硝基甲烷挤出反应合成多环苯并咪唑杂环。
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
报道了一种碱基促进的二胺与4-氯-3-硝基香豆素或4-氯-3-硝基喹啉-2(1H)- 1的环化反应,得到6- h -苯并咪唑[1,2-c][1,3]苯并恶嗪-6- 1或苯并[4,5]咪唑[1,2-c]喹唑啉-6(5H)- 1,产率中等至较高。这些无金属和三光气的反应可能是通过将二胺偶联加成到4-氯-3-硝基香豆素/4-氯-3-硝基喹啉-2(1H)-上,然后通过挤压硝基甲烷和氯化氢在分子内环化形成目标化合物进行的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
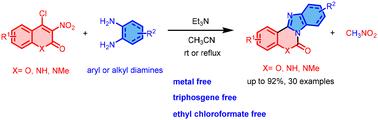
A nitromethane extrusion reaction for the synthesis of polycyclic benzimidazoheterocycles
A facile base-promoted annulation of diamines with 4-chloro-3-nitrocoumarins or 4-chloro-3-nitroquinolin-2(1H)-ones affording 6H-benzimidazo[1,2-c][1,3]benzoxazin-6-ones or benzo[4,5]imidazo[1,2-c]quinazolin-6(5H)-ones in moderate to good yields is reported. These metal- and triphosgene-free reactions presumably proceed by the conjugate addition of diamines to 4-chloro-3-nitrocoumarins/4-chloro-3-nitroquinolin-2(1H)-ones, followed by intramolecular cyclization via extrusion of nitromethane and hydrogen chloride to form the target compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


