具有二价组蛋白标记的复合转座子在细胞命运调控中发挥rna依赖性增强子的作用
IF 42.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
离散的基因组单位可以重组成复合转座子,这些转座子作为单个单位进行转录和转座,但它们的调控和功能尚不完全清楚。我们报道复合转座子含有双价组蛋白标记,在不同的区域具有激活和抑制标记。全基因组CRISPR-Cas9筛选,利用人类细胞中由古人类特异性复合转座子SVA (sin[短穿插核元件]-VNTR[可变数目串联重复序列]-Alu)驱动的报告基因,鉴定出多种修饰二价组蛋白标记以调节SVA转录的基因。SVA转录本是SVA选择性接触和激活远程基因表达的顺式调控功能的关键。值得注意的是,一组二价SVAs在红细胞生成过程中被激活,以促进多个红细胞基因的表达,而敲低这些SVAs会导致红细胞生成缺陷。SVA的rna依赖性顺式调节功能激活骨髓形成基因,并有助于衰老相关的骨髓偏向性造血。这些结果表明,复合转座子的顺式调控功能在发育和衰老过程中受到二价调控,从而控制细胞命运的转变。本文章由计算机程序翻译,如有差异,请以英文原文为准。
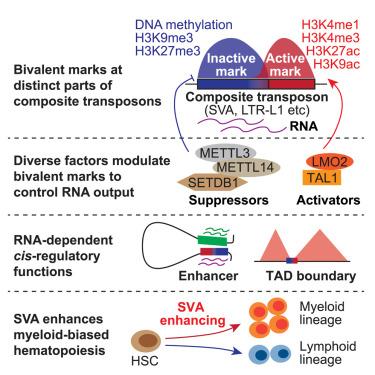
Composite transposons with bivalent histone marks function as RNA-dependent enhancers in cell fate regulation
Discrete genomic units can recombine into composite transposons that transcribe and transpose as single units, but their regulation and function are not fully understood. We report that composite transposons harbor bivalent histone marks, with activating and repressive marks in distinct regions. Genome-wide CRISPR-Cas9 screening, using a reporter driven by the hominid-specific composite transposon SVA (SINE [short interspersed nuclear element]-VNTR [variable number of tandem repeats]-Alu) in human cells, identified diverse genes that modify bivalent histone marks to regulate SVA transcription. SVA transcripts are critical for SVA’s cis-regulatory function in selectively contacting and activating long-range gene expression. Remarkably, a subset of bivalent SVAs is activated during erythropoiesis to boost multiple erythroid gene expression, and knocking down these SVAs leads to deficient erythropoiesis. The RNA-dependent cis-regulatory function of SVA activates genes for myelopoiesis and can contribute to aging-associated myeloid-biased hematopoiesis. These results reveal that the cis-regulatory functions of composite transposons are bivalently regulated to control cell fate transitions in development and aging.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


