智能微流控装置用于上呼吸道和下呼吸道感染的多重检测和及时预警
IF 13
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
多种呼吸道疾病的发病率和合并感染率不断上升,迫切需要高效、可靠的呼吸道病原体多重诊断方法。目的研制一种用于呼吸道感染监测和预警的多功能诊断工具。方法研制了一种便携式、离心式、高度集成的实时重组酶辅助扩增(RAA)微流控装置,用于15种常见呼吸道病原体的特异性鉴定。使用模拟拭子和含有15种病原体的痰液验证了其在护理点的应用。采用传统的提取试剂盒和RT-qPCR/qPCR方法,共收集427份样品,评估其临床性能。此外,其互联监测和预警平台的可行性在中国大陆8个城市的22个场景中进行了验证。结果该装置通过自动核酸提取、实时RAA、荧光检测和无线数据传输,成功实现了从样品到答案的诊断。与传统方法相比,该设备在周转时间(拭子30 min,痰液60 min)和单次检测可检测目标/类型方面具有明显优势。该装置对病毒靶点的最低检出限为0.1 TCID50/mL,对细菌或真菌的最低检出限为10 CFU/mL。这种新开发的设备在检测临床样品中各种类型的病原体(病毒、细菌和真菌)方面具有良好的通用性,在345份拭子和82份痰样本的分析中,所有目标的AUC均高于0.99,准确率为99.30 %。此外,该设备实现了与智能监控预警系统跨22个场景的无缝数据交互,成功实现了上传信息的数据统计和预警分析可视化。结论该装置为多种病原菌的早期诊断提供了一个智能、便携、快速、灵敏的平台,尤其适用于资源有限的地区。本文章由计算机程序翻译,如有差异,请以英文原文为准。
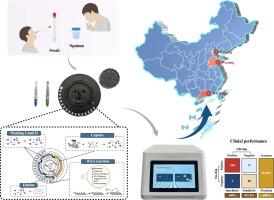
Intelligent microfluidic device for multiplex detection and prompt warning of upper and lower respiratory tract infections
Introduction
The increasing incidence and co-infection rates of multiple respiratory diseases have emphasized the necessity for efficient and reliable multiplex diagnostic methods for respiratory pathogens.Objective
This study aims to develop a multiplex diagnostic tool for monitoring and early warning of respiratory infections.Methods
A portable, centrifugal and highly integrated microfluidic device based on real-time recombinase-aided amplification (RAA) assays capable of specifically identifying 15 common respiratory pathogens was developed. Its point-of-care application was validated using simulated swabs and sputum spiked with 15 pathogens. A total of 427 collected samples were employed to assess its clinical performance, in parallel with traditional extraction kits and RT-qPCR/qPCR assays. Additionally, the feasibility of its connected monitoring and early warning platform was validated across 22 scenarios in 8 cities of mainland China.Results
This device successfully facilitated sample-to-answer diagnostics by automating nucleic acid extraction, real-time RAA, fluorescence detection and wireless data transmission. Compared with conventional methods, this device has obvious advantages in the turnaround time (30 min for swabs and 60 min for sputum) and the detectable targets/types in a single test. The lowest detection limits of this device were 0.1 TCID50/mL for viral targets and 10 CFU/mL for bacterial or fungal targets. This newly developed device has good versatility in detecting various types of pathogens (viruses, bacteria, and fungi) in clinical samples, achieving an AUC above 0.99 for all targets and an accuracy of 99.30 % in the analysis of 345 swab and 82 sputum samples. Moreover, this device achieved seamless data interaction with the intelligent monitoring and early warning system across 22 scenarios, enabling the successful visualization of data statistics and early warning analyses for uploaded information.Conclusion
This device provides an intelligent, portable, rapid, and sensitive platform for early diagnosis of multiple pathogens, particularly in resource-limited settings.求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


