阐明细胞色素P450酶的底物特异性:对含N和s的小分子代谢的见解
IF 11.6
1区 工程技术
Q1 ENGINEERING, MULTIDISCIPLINARY
引用次数: 0
摘要
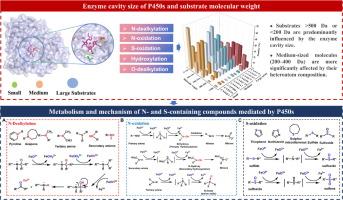
细胞色素P450酶(P450或CYPs)是小分子药物吸收、分布、代谢和排泄(ADME)特性的主要代谢贡献者。这些酶可以催化各种类型的反应,包括发生在小分子氮(N)和硫(S)位点的代谢反应。在这篇综述中,我们对294种p450s介导的小分子底物进行了全面的统计分析,其中含有N和s的底物超过47%,分析的目的是阐明这些底物与5种反应类型的各种CYP异构体之间的广谱交叉反应性和特异性。我们的研究结果表明,分子量大于500 Da或小于200 Da的底物主要受CYP异构体活性位点的主导作用支配。相比之下,分子量在200 - 400 Da之间的中小分子对其所含杂原子的类型有更强的依赖性,酶的催化位点(空腔)的大小在决定底物特异性方面的作用可以忽略不计。本文从P450s介导的含N和含s化合物的代谢机制出发,系统分析了N-脱烷基、N-氧化和s-氧化所涉及底物的结构特征及其与P450s的代谢相互作用。这些分析为进一步了解P450s底物特异性与底物结构特征之间的关系提供了新的视角,并为基于P450s催化反应框架加强药物设计和预测代谢稳定性提供了有价值的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Elucidating the Substrate Specificity of Cytochrome P450 Enzymes: Insights into N- and S-Containing Small-Molecule Metabolism
Cytochrome P450 enzymes (P450s or CYPs) are the primary metabolic contributors to the absorption, distribution, metabolism, and excretion (ADME) properties of small-molecule drugs. These enzymes can catalyze various types of reactions, including metabolic reactions that occur at nitrogen (N) and sulfur (S) sites of small molecules. In this review, we conducted a comprehensive statistical analysis of 294 P450s-mediated small-molecule substrates, among which more than 47% substrates contained N and S. The purpose of the analysis is to elucidate the broad-spectrum cross-reactivity and specificity between these substrates and various CYP isoforms across five reaction types. Our findings reveal that substrates with molecular weights greater than 500 Da or less than 200 Da are predominantly governed by the dominant effect of the CYP isoform’s active sites. In contrast, small- to medium-sized molecules with molecular weights ranging from 200 to 400 Da exhibit a stronger dependence on the types of heteroatoms they contain, with the size of the enzyme’s catalytic site (cavity) playing a negligible role in determining substrate specificity. This review starts from the metabolic mechanisms of P450s-mediated N- and S-containing compounds, and systematically analyzes the structural characteristics of substrates involved in N-dealkylation, N-oxidation, and S-oxidation, as well as their metabolic interactions with P450s. These analyses provide a new perspective for improving the existing understanding of the relationship between the P450s substrate specificity and substrate structural characteristics, and offer a valuable perspective for enhancing drug design and predicting metabolic stability based on the P450s-catalyzed reaction framework.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Engineering
Environmental Science-Environmental Engineering
自引率
1.60%
发文量
335
审稿时长
35 days
期刊介绍:
Engineering, an international open-access journal initiated by the Chinese Academy of Engineering (CAE) in 2015, serves as a distinguished platform for disseminating cutting-edge advancements in engineering R&D, sharing major research outputs, and highlighting key achievements worldwide. The journal's objectives encompass reporting progress in engineering science, fostering discussions on hot topics, addressing areas of interest, challenges, and prospects in engineering development, while considering human and environmental well-being and ethics in engineering. It aims to inspire breakthroughs and innovations with profound economic and social significance, propelling them to advanced international standards and transforming them into a new productive force. Ultimately, this endeavor seeks to bring about positive changes globally, benefit humanity, and shape a new future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


