邻苯二胺:一种可切换和生物正交共轭的潜在战斗部
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
先前作为光亲和标记(PAL)标签报道的4-叠氮酞酰亚胺被重新用作后偶联战斗部。利用邻苯二胺-胺反应,菁胺被成功地偶联到PAL后的靶蛋白上,随后被其他胺取代,证明了其“可切换”的性质。这些发现突出了邻苯二胺作为一种新的生物正交偶联化学工具的独特特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
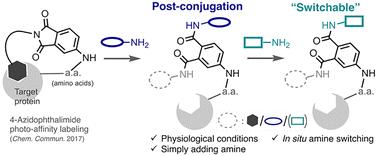
Phthalimide: a potential warhead for switchable and bioorthogonal conjugation
4-Azidophthalimide, previously reported as a photoaffinity labeling (PAL) tag, was repurposed as a post-conjugation warhead. Using the phthalimide–amine reaction, cyanine amines were successfully conjugated to target proteins after PAL and later replaced with other amines, demonstrating its “switchable” nature. These findings highlight phthalimide's unique characteristics as a novel chemical tool for bioorthogonal conjugations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


