电场辅助纳滤对锂镁高效分离的机理研究
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
锂作为新能源领域的关键资源,对高效节能的锂镁分离技术提出了更高的要求。本研究采用电场辅助纳滤(E-NF)技术实现锂镁的高效分离。与常规纳滤相比,在电流密度为2 mA·cm-2时,Li +的截留率从-27.4%下降到-32%,而Mg2+的截留率从95.8%上升到99%。利用operando拉曼光谱、x射线散射(XRS)和分子动力学(MD)模拟探索了潜在的分子机制。结果表明,电场在LiCl溶液中比在MgCl2溶液中更明显地破坏了氢键,导致锂离子周围的散装水结构更松散。XRS和拉曼数据显示,低ow和离子-水相互作用的峰值强度降低,在LiCl溶液中效果更明显。Li-O和Mg-O特征峰强度的降低表明离子与水分子之间的相互作用减弱。MD模拟表明,电场通过减少Li +的配位壳增强了Li +的脱水和膜渗透性,而对Mg2+的影响很小。总的来说,电场加速了Li +的脱水,促进了Li +快速通过膜,而Mg +通过Donnan效应和介电排斥被保留,有效地将Li +和Mg +分离开来。本文章由计算机程序翻译,如有差异,请以英文原文为准。
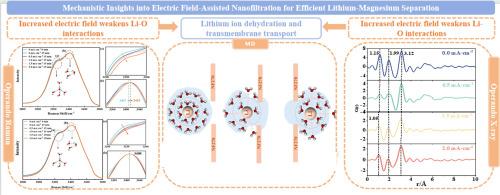
Mechanistic Insights into Electric Field-Assisted Nanofiltration for Efficient Lithium-Magnesium Separation
Lithium, a pivotal resource in the new energy sector, demands the development of efficient and energy-saving lithium-magnesium separation technologies. This study employed electric field-assisted nanofiltration (E-NF) technology to achieve efficient lithium-magnesium separation. Compared with conventional nanofiltration, at a current density of 2 mA·cm-2, the rejection rate of Li⁺ decreased from -27.4% to -32%, while that of Mg2+ increased from 95.8% to 99%. The underlying molecular mechanisms were explored using operando Raman spectroscopy, X-ray scattering (XRS), and molecular dynamics (MD) simulations. Results indicate that the electric field disrupts hydrogen bonds in LiCl solutions more significantly than in MgCl2 solutions, leading to a looser bulk water structure around lithium ions. XRS and Raman data show reduced peak intensities for Ow-Ow and ion-water interactions, with more pronounced effects in LiCl solutions. The decrease in Li-O and Mg-O characteristic peak intensities indicates a weakening interaction between ions and water molecules. MD simulations reveal that the electric field enhances Li⁺ dehydration and membrane permeation by reducing its coordination shells, while only slightly affecting Mg2+. Overall, the electric field accelerates Li⁺ dehydration, facilitating its rapid passage through the membrane, while Mg²⁺ is retained via Donnan effects and dielectric exclusion, effectively separating Li⁺ from Mg²⁺.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


