肿瘤进展过程中细胞外囊泡活性的机械调节
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
细胞外囊泡(EVs)是由细胞分泌的自然形成的膜结合囊泡。电动汽车具有表面靶向分子的功能,并携带信号蛋白和核酸作为货物,可以重新连接途径并改变受体细胞的生物过程。肿瘤源性ev通过促进肿瘤细胞侵袭和转移前生态位的建立,在癌症进展,特别是转移中发挥关键作用。对肿瘤中ev的不断发展的理解强调了肿瘤微环境内外复杂的细胞间通讯网络,涉及癌细胞和非癌细胞类型,如成纤维细胞和内皮细胞。最近,EVs也被认为在调节宿主和免疫细胞之间的相互作用以及重新编程肿瘤免疫微环境中发挥作用。在这篇综述中,我们讨论了EV在不同的机械生物学和机械免疫学背景下的生物发生和功能,强调了机械线索如何影响EV的靶向和活性。机械力和EV动力学之间复杂的相互作用有助于肿瘤进展,并将EV与关键疾病特征联系起来。本文章由计算机程序翻译,如有差异,请以英文原文为准。
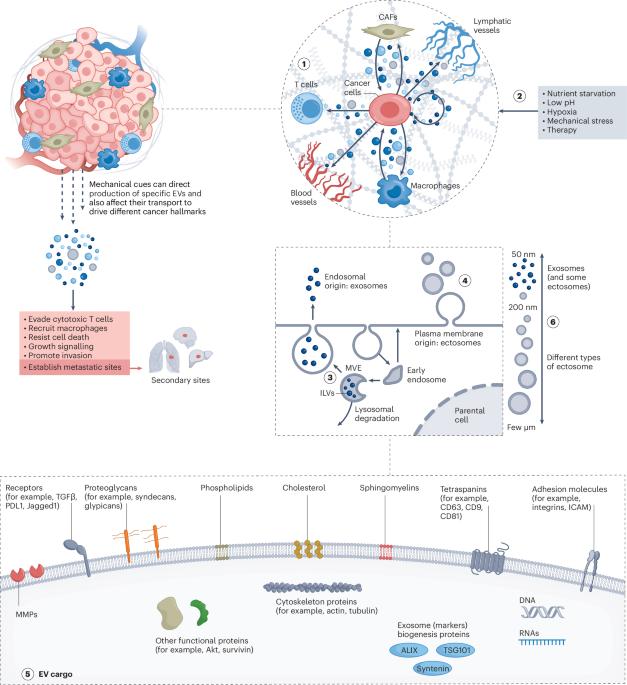
Mechanical regulation of extracellular vesicle activity during tumour progression
Extracellular vesicles (EVs) are naturally occurring membrane-bound vesicles secreted by cells. Functionalized with surface-targeting molecules and carrying signalling proteins and nucleic acids as cargo, EVs can rewire pathways and alter biological processes in recipient cells. Tumour-derived EVs have key roles in cancer progression, particularly in metastasis, by promoting tumour cell invasion and the establishment of pre-metastatic niches. An evolving understanding of EVs in cancer highlights a complex intercellular communication network within and beyond the tumour microenvironment that involves cancer cells and non-cancerous cell types, such as fibroblasts and endothelial cells. More recently, EVs have also been recognized for their role in modulating interactions between host and immune cells and in reprogramming the tumour immune microenvironment. In this Review, we discuss EV biogenesis and function in diverse mechanobiological and mechanoimmunological contexts, highlighting how mechanical cues influence EV targeting and activity. The intricate interplay between mechanical forces and EV dynamics contributes to tumour progression and links EVs to key disease hallmarks. This Review discusses how mechanical cues can influence extracellular vesicle targeting and activity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


