碱性电解质中PEO预击穿阶段Mg的阳极行为
IF 13.8
1区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
摘要
镁的等离子体电解氧化(PEO)的电解质选择非常重要,因为这决定了所得到的涂层的组成、形态和性能,这是镁合金在恶劣环境中免受腐蚀和磨损的迫切需要。然而,电解质设计通常是启发式的,这阻碍了新的PEO工艺的开发和优化。在这里,我们试图从电化学和微观结构方面对镁在NaAlO2、Na3PO4、NaF和Na2SiO3的碱性水溶液中PEO处理预击穿阶段阳极膜演变的机理进行理解。系统的研究表明,MgO/Mg(OH)2对镁的自钝化会受到阳极反应副作用引起的化学和机械不稳定性的影响。PEO过程的稳定启动需要在广泛的pH范围内保持表面钝化,这只能通过将自沉积钝化剂与将溶解的镁结合成不溶性化合物的钝化剂结合来实现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
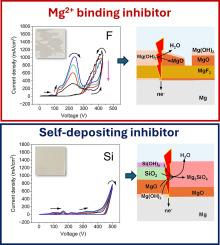
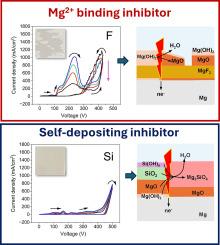
Anodic behaviour of Mg at pre-breakdown stages of PEO in basic electrolytes
Electrolyte selection for Plasma Electrolytic Oxidation (PEO) of magnesium is important as this determines composition, morphology and properties of resultant coatings that are urgently sought after for protection of Mg alloys from corrosion and wear in harsh environments. However, electrolyte design is often performed heuristically, which hampers the development and optimisation of new PEO processes. Here, we attempt to achieve a mechanistic understanding of electrochemical and microstructural aspects of anodic films evolution at the pre-breakdown stages of PEO treatments of magnesium in aqueous alkaline solutions of NaAlO2, Na3PO4, NaF and Na2SiO3. Systematic studies have shown that magnesium self-passivation by MgO/Mg(OH)2 can be compromised by both chemical and mechanical instabilities developed due to side effects of anodic reactions. Stable initiation of PEO process requires maintaining surface passivity in a wide range of pH, which can be achieved only by combining self-depositing passivators with those binding dissolved magnesium into insoluble compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Magnesium and Alloys
Engineering-Mechanics of Materials
CiteScore
20.20
自引率
14.80%
发文量
52
审稿时长
59 days
期刊介绍:
The Journal of Magnesium and Alloys serves as a global platform for both theoretical and experimental studies in magnesium science and engineering. It welcomes submissions investigating various scientific and engineering factors impacting the metallurgy, processing, microstructure, properties, and applications of magnesium and alloys. The journal covers all aspects of magnesium and alloy research, including raw materials, alloy casting, extrusion and deformation, corrosion and surface treatment, joining and machining, simulation and modeling, microstructure evolution and mechanical properties, new alloy development, magnesium-based composites, bio-materials and energy materials, applications, and recycling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


