达格列净相关的肝毒性与阳性再挑战。
IF 0.5
Q4 GASTROENTEROLOGY & HEPATOLOGY
ACG Case Reports Journal
Pub Date : 2025-08-04
eCollection Date: 2025-08-01
DOI:10.14309/crj.0000000000001799
引用次数: 0
摘要
葡萄糖共转运蛋白2抑制剂钠通常是安全的药物,有糖尿病、心力衰竭和慢性肾脏疾病的适应症。我们报告一例疑似达格列净引起的肝损伤,患者为68岁男性,既往不明原因肝炎发作后出现2周的上腹部疼痛、肌痛和转氨炎。经修订的电子因果关系评估方法(RECAM)评分为9分(极有可能),确定患者肝损伤伴黄疸可能是由达格列净引起的。在随访期间停用达格列净后,实验室结果和临床症状得到缓解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
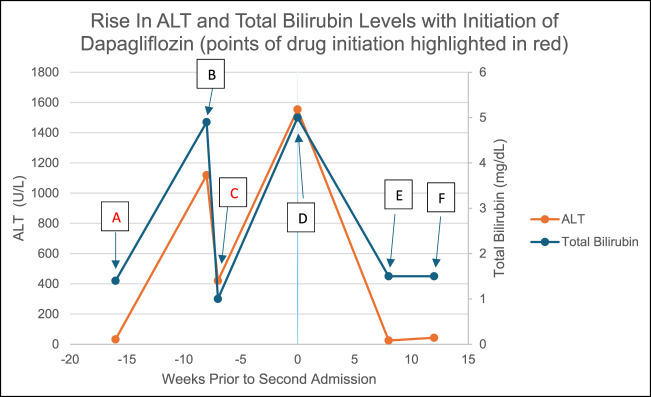
Dapagliflozin-Associated Hepatotoxicity With a Positive Rechallenge.
Sodium glucose cotransporter 2 inhibitors are generally safe medications, with labelled indications for diabetes mellites, heart failure, and chronic kidney disease. We present a case of suspected dapagliflozin-induced liver injury in a 68-year-old man who presented with 2 weeks of upper abdominal pain, myalgias, and transaminitis following a prior episode of unexplained hepatitis. It was determined that the patient's liver injury with jaundice was likely due to dapagliflozin with a Revised Electronic Causality Assessment Method (RECAM) score of 9 (Highly likely). Laboratory findings and clinical symptoms resolved after discontinuing dapagliflozin during follow up.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACG Case Reports Journal
GASTROENTEROLOGY & HEPATOLOGY-
自引率
14.30%
发文量
170
审稿时长
12 weeks
期刊介绍:
ACG Case Reports Journal is a peer-reviewed, open-access publication that provides GI and hepatology fellows, private practice clinicians, and other healthcare providers an opportunity to share interesting case reports with their peers and with leaders in the field. ACG Case Reports Journal publishes case reports, images, videos and letters to the editor in all topics of gastroenterology and hepatology, including: Biliary Colon Endoscopy Esophagus Functional Bowel Disorders Inflammatory Bowel Disease Liver Nutrition and Obesity Pancreas Pathology Pediatric Small Bowel Stomach.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


