生物启发合成阿比索菌素C的内酰胺类似物
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
Sorensen开发的生物启发方法已被用于制备含有α, β-不饱和内酰胺的新型类似物。该合成旨在利用分子内Diels-Alder反应在后期合成中提供含有深层修饰的类似物,以探索其抗菌性能。总体而言,安装这种非天然内酰胺片段是成功的,并允许对类似物进行抗菌评估,但低产量突出了利用生物启发方法获得新的自然分子靶标的常见挑战。本文章由计算机程序翻译,如有差异,请以英文原文为准。
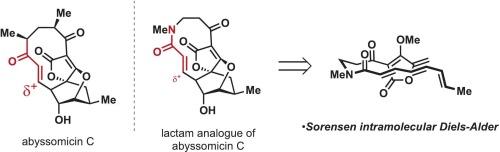
Bioinspired synthesis of a lactam analogue of abyssomicin C
The bioinspired approach to abyssomicin C developed by Sorensen has been utilized to prepare a novel analog containing an α, β-unsaturated lactam. This synthesis was designed to utilize the intramolecular Diels-Alder reaction in late-stage synthesis to provide analogs that contain deep-seated modifications to explore their antimicrobial properties. Overall, installing this non-natural lactam moiety was successful and allowed for antimicrobial assessment of the analog, but the low yields highlight the frequent challenges of leveraging a bioinspired approach for a new-to-nature molecular target.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


