硫辛酸修饰的表柔比星脂质体系统用于肿瘤靶向药物传递和心脏毒性降低
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-08-05
DOI:10.1016/j.bioadv.2025.214439
引用次数: 0
摘要
本研究旨在通过构建表柔比星硫辛酸功能化脂质体(Epi Lip@LA)来解决蒽环类药物递送效率低、心脏毒性高的瓶颈问题。该系统通过硫辛酸修饰被赋予动态靶向和微环境响应的双重功能:DSPE-PEG2000-LA中的硫辛酸基团通过动态共价键介导的靶向递送增强肿瘤细胞的摄取效率,肿瘤微环境中高浓度的谷胱甘肽(GSH)触发二硫键的特异性裂解,实现药物的精准释放。同时,硫辛酸通过清除活性氧、维持线粒体膜电位稳定、抑制NLRP3炎性小体活化,协同阻断心肌氧化应激和炎症损伤。体内研究表明,该系统在显著增强抗肿瘤疗效的同时,有效缓解心肌纤维化等病理改变,实现了给药效率和心脏安全性的双重优化。这项工作为开发具有高效肿瘤靶向和系统保护功能的纳米载体系统提供了一种创新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
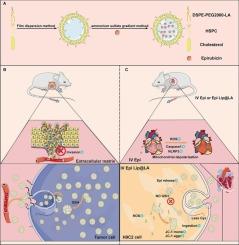
Lipoic acid-modified epirubicin liposomal system for tumor-targeted drug delivery and cardiotoxicity reduction
This study aimed to address the bottlenecks of low delivery efficiency and high cardiotoxicity of anthracyclines by constructing liposomes of epirubicin functionalized with lipoic acid (Epi Lip@LA). This system was endowed with dual functions of dynamic targeting and microenvironmental response through lipoic acid modification: the lipoic acid group in DSPE-PEG2000-LA enhanced the uptake efficiency of tumor cells through dynamic covalent bond-mediated targeted delivery, and the high concentration of glutathione (GSH) in the tumor microenvironment triggered the specific cleavage of disulfide bonds to achieve precise drug release. Meanwhile, lipoic acid cooperatively blocked myocardial oxidative stress and inflammatory injury by scavenging reactive oxygen species, maintaining mitochondrial membrane potential stability, and inhibiting NLRP3 inflammasome activation. In vivo studies demonstrated that this system significantly enhanced the anti-tumor efficacy while effectively alleviating pathological changes such as myocardial fibrosis, achieving dual optimization of drug delivery efficiency and cardiac safety. This work provides an innovative strategy for developing nanocarrier systems with both efficient tumor targeting and systemic protective functions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


