新出现的细胞因子有助于糖尿病患者颈动脉斑块形成和稳定的临床表现
IF 2.1
Q3 PERIPHERAL VASCULAR DISEASE
引用次数: 0
摘要
背景2型糖尿病(T2DM)通过慢性炎症和免疫失调促进动脉粥样硬化和斑块不稳定。新出现的证据表明,白细胞介素-1家族的成员,特别是IL-36、IL-37和il -38,参与了糖尿病和非糖尿病血管疾病炎症反应的调节。然而,它们在颈动脉粥样硬化中的作用仍不明确。方法采用ELISA法对20例2型糖尿病患者和40例非糖尿病患者的循环细胞因子水平进行回顾性观察。从动脉内膜切除术中收集颈动脉斑块样本(n = 50),并根据AHA标准将其分为稳定(无症状,n = 25)或不稳定(有症状,n = 25)。免疫组织化学定量IL-36α、IL-36β、IL-36γ、IL-37和IL-38的局部表达。结果T2DM患者外周血IL-36α、IL-36β、IL-36γ水平明显升高,IL-37、IL-38水平无明显变化。免疫组织化学显示,在不稳定斑块和稳定斑块中,所有五种细胞因子的表达显著增加。按糖尿病状态分层显示,这种上调是糖尿病患者独有的。值得注意的是,IL-36β在不稳定的糖尿病斑块中表现出最明显的增加,超过25倍。IL-37和IL-38也在局部升高,可能反映了代偿性抗炎反应,尽管它们的循环水平未受影响。结论:IL-36、IL-37和IL-38在颈动脉粥样硬化斑块中的表达存在差异,IL-36β在糖尿病患者中是促动脉粥样硬化的关键细胞因子。这些细胞因子在糖尿病斑块和非糖尿病斑块中的不同表达模式表明,T2DM加重了动脉粥样硬化中的免疫失衡。这些发现可能为未来的细胞因子靶向治疗糖尿病血管并发症的研究提供信息。本文章由计算机程序翻译,如有差异,请以英文原文为准。
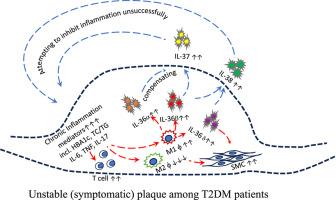
Emerging cytokines contribute to the clinical manifestation of carotid artery plaque formation and stability in patients with diabetes
Background
Type 2 diabetes mellitus (T2DM) promotes atherosclerosis and plaque instability through chronic inflammation and immune dysregulation. Emerging evidence implicates members of the interleukin-1 family—particularly IL-36, IL-37, and IL-38—in the modulation of inflammatory responses in diabetic and non-diabetic vascular disease. However, their roles in carotid atherosclerosis remain poorly defined.
Methods
In this retrospective observational study, circulating cytokine levels were assessed in 20 T2DM and 40 non-DM individuals using ELISA. Carotid plaque samples (n = 50) were collected from endarterectomy procedures and categorised as stable (asymptomatic, n = 25) or unstable (symptomatic, n = 25) according to AHA criteria. Immunohistochemistry quantified local expression of IL-36α, IL-36β, IL-36γ, IL-37, and IL-38.
Results
Circulating IL-36α, IL-36β and IL-36γ were significantly elevated in T2DM patients, while IL-37 and IL-38 levels were unchanged. Immunohistochemistry revealed significantly increased expression of all five cytokines in unstable versus stable plaques. Stratification by diabetic status showed that this upregulation was exclusive to diabetic patients. Notably, IL-36β exhibited the most pronounced increase—over 25-fold—in unstable diabetic plaques. IL-37 and IL-38 were also elevated locally, likely reflecting compensatory anti-inflammatory responses, though their circulating levels remained unaffected.
Conclusions
This study demonstrates differential expression of IL-36, IL-37, and IL-38 in carotid atherosclerotic plaques, with IL-36β emerging as a key pro-atherogenic cytokine in the diabetic setting. The distinct expression patterns of these cytokines in diabetic versus non-diabetic plaques suggest that T2DM exacerbates immune imbalance in atherosclerosis. These findings may inform future research on cytokine-targeted therapies for diabetic vascular complications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Atherosclerosis plus
Cardiology and Cardiovascular Medicine
CiteScore
2.60
自引率
0.00%
发文量
0
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


