甘露聚糖对法菲小鹿精发酵乳蛋白下游加工的干扰及纯化效率
IF 8.2
Q1 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
从Komagataella phaffii中精确发酵乳蛋白是一项成熟的技术,但高下游加工成本仍然具有挑战性。本研究基于蛋白质和非蛋白质含量对细胞外分泌重组β-乳球蛋白(rBLG)、未磷酸化αs1-酪蛋白(rCSN)和乳铁蛋白(rLTF)进行了表征,并将它们与动物源性的对应物进行了比较。对三种纯化方法进行了评价。两种是基于电荷的,即rBLG和rCSN的等电点(IEP)沉淀在pH范围为2 ~ 7.5,rLTF和阴离子交换(AEX)色谱在5.5 ~ 11;一个是基于尺寸的膜分离。所有的靶蛋白在二级结构上与它们在动物基础上的对应物匹配约95%。与蛋白质无关,甘露聚糖(52-66% d.b.a, 2-242 kDa, 75-87%甘露糖)是主要杂质。由于蛋白质和甘露聚糖的大小相似,基于尺寸的膜分离效果不佳。基于收费的方法更为成功。AEX能有效去除甘露聚糖,将甘露聚糖的蛋白质纯度从20-41%提高到64-81%,但仅回收32-37%的蛋白质,限制了其在食品工业中的应用。IEP沉淀法仅适用于rCSN,最终蛋白纯度高达77%(沉淀部分),只有7%的酪蛋白未沉淀。未来的工作应侧重于更好地去除甘露聚糖,以满足功能应用的纯度要求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
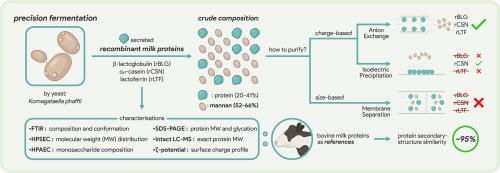
Mannan interference and purification efficiency in downstream processing of precision-fermented milk proteins from Komagataella phaffii
Precision-fermented milk proteins from Komagataella phaffii are a well-established technology, but high downstream processing costs remain challenging. This study characterised extracellularly secreted recombinant β-lactoglobulin (rBLG), unphosphorylated αs1-casein (rCSN), and lactoferrin (rLTF) based on protein and non-protein content, comparing them to their animal-derived counterparts. Three purification methods were evaluated. Two were charge-based, i.e., isoelectric point (IEP) precipitation at a pH range of 2 to 7.5 for rBLG and rCSN and 5.5 to 11 for rLTF and anion-exchange (AEX) chromatography; one was size-based membrane separation. All target proteins matched ∼95% of their animal-based counterparts in secondary structure. Irrespective of the protein, mannans (52–66% d.b., 2–242 kDa, 75–87% mannose) were the main impurity. Size-based membrane separation was ineffective due to the similar sizes of protein and mannan. Charge-based methods were more successful. AEX removed mannan effectively, increasing the protein purity from 20–41% to 64–81%, but recovered only 32–37% protein, limiting its use in the food industry. IEP precipitation worked only for rCSN, obtaining final protein purity up to 77% (in precipitated fraction) with only 7% of the casein remaining unprecipitated. Future work should focus on better mannan removal to meet purity demands for functional applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Future Foods
Agricultural and Biological Sciences-Food Science
CiteScore
8.60
自引率
0.00%
发文量
97
审稿时长
15 weeks
期刊介绍:
Future Foods is a specialized journal that is dedicated to tackling the challenges posed by climate change and the need for sustainability in the realm of food production. The journal recognizes the imperative to transform current food manufacturing and consumption practices to meet the dietary needs of a burgeoning global population while simultaneously curbing environmental degradation.
The mission of Future Foods is to disseminate research that aligns with the goal of fostering the development of innovative technologies and alternative food sources to establish more sustainable food systems. The journal is committed to publishing high-quality, peer-reviewed articles that contribute to the advancement of sustainable food practices.
Abstracting and indexing:
Scopus
Directory of Open Access Journals (DOAJ)
Emerging Sources Citation Index (ESCI)
SCImago Journal Rank (SJR)
SNIP
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


