纳米Fe3O4在毫米级阴离子交换树脂中的原位约束增强双酚A吸附
IF 5.1
3区 工程技术
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
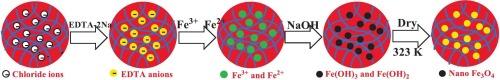
双酚A (BPA)是一种雌激素和内分泌干扰物,广泛存在于地下水、地表水甚至饮用水中。本文通过原位约束Fe3O4纳米晶在毫米级D201阴离子交换树脂中成功制备了磁性D201-Fe3O4纳米复合材料。D201-Fe3O4纳米复合材料的Fe元素能谱图、TEM图像、XRD图谱和VSM磁化曲线显示,约束态Fe3O4颗粒分布均匀、纳米尺寸、高结晶性和强超顺磁性。得益于聚合物骨架和Fe3O4纳米晶的协同作用,D201-Fe3O4纳米复合材料具有良好的热稳定性和化学稳定性。D201-Fe3O4纳米复合材料对BPA的最大吸附量为246.9 mg/g,超过了大多数文献报道的吸附剂。在3.8 ~ 9.8的较宽pH范围内,D201-Fe3O4纳米复合材料对BPA的吸附能力基本不变。与D201相比,当吸附0.7 mg/mL BPA溶液时,额外的OFe配位键使D201- fe3o4纳米复合材料的一级速率常数k1增加2.9倍,可处理体积增加1.4倍。D201-Fe3O4纳米复合材料在80% c2h5oh - 1.5% NaCl二元溶液中易于更新,经过5次吸附-解吸循环后具有良好的重复使用性。D201-Fe3O4纳米复合材料有望在大规模应用中去除BPA。本文章由计算机程序翻译,如有差异,请以英文原文为准。
In situ confinement of Fe3O4 nanocrystalline in millimeter-sized anion exchange resin for enhanced bisphenol A adsorption
Bisphenol A (BPA), as an estrogen and endocrine disruptor, has been extensively found in groundwater, surface water and even drinking water. Herein, the magnetic D201-Fe3O4 nanocomposite was successfully fabricated through in situ confinement of Fe3O4 nanocrystalline in millimeter-sized D201 anion exchange resin. EDS elemental mapping of Fe, TEM image, XRD pattern and VSM magnetization curve of D201-Fe3O4 nanocomposite revealed that the confined Fe3O4 particles exhibited uniform distribution, nanosize, highly crystalline nature and strong superparamagnetism. Benefiting from the synergism of the polymer skeleton and Fe3O4 nanocrystalline, D201-Fe3O4 nanocomposite featured satisfactory thermal stability and chemical stability. D201-Fe3O4 nanocomposite showed the maximum BPA adsorption capacity of 246.9 mg/g, which exceeded most of the reported adsorbents in the literature. Within the wide pH range of 3.8–9.8, the adsorption capacities of BPA on D201-Fe3O4 nanocomposite were basically unchanged. Compared with D201, the additional O![]() Fe coordination bond made D201-Fe3O4 nanocomposite exhibit 2.9 times of the first order rate constant k1 and 1.4 times of the treatable volume when 0.7 mg/mL BPA solution was adsorbed. D201-Fe3O4 nanocomposite could be easily refreshed using binary 80 % C2H5OH-1.5 % NaCl solution and featured excellent reusability after five adsorption-desorption cycles. D201-Fe3O4 nanocomposite is promising for the removal of BPA in scaled-up application.
Fe coordination bond made D201-Fe3O4 nanocomposite exhibit 2.9 times of the first order rate constant k1 and 1.4 times of the treatable volume when 0.7 mg/mL BPA solution was adsorbed. D201-Fe3O4 nanocomposite could be easily refreshed using binary 80 % C2H5OH-1.5 % NaCl solution and featured excellent reusability after five adsorption-desorption cycles. D201-Fe3O4 nanocomposite is promising for the removal of BPA in scaled-up application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Reactive & Functional Polymers
工程技术-高分子科学
CiteScore
8.90
自引率
5.90%
发文量
259
审稿时长
27 days
期刊介绍:
Reactive & Functional Polymers provides a forum to disseminate original ideas, concepts and developments in the science and technology of polymers with functional groups, which impart specific chemical reactivity or physical, chemical, structural, biological, and pharmacological functionality. The scope covers organic polymers, acting for instance as reagents, catalysts, templates, ion-exchangers, selective sorbents, chelating or antimicrobial agents, drug carriers, sensors, membranes, and hydrogels. This also includes reactive cross-linkable prepolymers and high-performance thermosetting polymers, natural or degradable polymers, conducting polymers, and porous polymers.
Original research articles must contain thorough molecular and material characterization data on synthesis of the above polymers in combination with their applications. Applications include but are not limited to catalysis, water or effluent treatment, separations and recovery, electronics and information storage, energy conversion, encapsulation, or adhesion.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


