葡萄糖依赖性鞘糖脂生物合成促进CD8+ T细胞功能和肿瘤控制
IF 30.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
葡萄糖对T细胞的增殖和功能至关重要,但其在体内的具体代谢作用仍不清楚。在这里,我们确定鞘糖脂(GSL)的生物合成是葡萄糖促进CD8+ T细胞扩增和体内细胞毒性功能的关键途径。利用基于13c的稳定同位素示踪,我们证明CD8+效应T细胞利用葡萄糖合成尿苷二磷酸葡萄糖(UDP-Glc),这是糖原、聚糖和GSL生物合成的前体。通过靶向酶UDP-Glc焦磷酸化酶2 (UGP2), udp - gal -4- epimase (GALE)或UDP-Glc神经酰胺葡萄糖基转移酶(UGCG)抑制GSL的产生可损害CD8+ T细胞在病原体攻击下的扩增。在机制上,我们发现葡萄糖依赖的GSL生物合成是质膜脂筏完整性和最佳T细胞受体(TCR)信号传导所必需的。此外,ugcg缺陷的CD8+ T细胞在体内表现出颗粒酶表达、细胞溶解活性和肿瘤控制的降低。总之,我们的数据表明GSL生物合成是葡萄糖的关键代谢命运-超越能量生产-这是体内CD8+ T细胞反应所必需的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
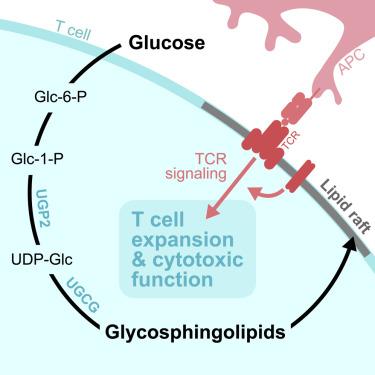
Glucose-dependent glycosphingolipid biosynthesis fuels CD8+ T cell function and tumor control
Glucose is essential for T cell proliferation and function, yet its specific metabolic roles in vivo remain poorly defined. Here, we identify glycosphingolipid (GSL) biosynthesis as a key pathway fueled by glucose that enables CD8+ T cell expansion and cytotoxic function in vivo. Using 13C-based stable isotope tracing, we demonstrate that CD8+ effector T cells use glucose to synthesize uridine diphosphate-glucose (UDP-Glc), a precursor for glycogen, glycan, and GSL biosynthesis. Inhibiting GSL production by targeting the enzymes UDP-Glc pyrophosphorylase 2 (UGP2), UDP-Gal-4-epimerase (GALE), or UDP-Glc ceramide glucosyltransferase (UGCG) impairs CD8+ T cell expansion upon pathogen challenge. Mechanistically, we show that glucose-dependent GSL biosynthesis is required for plasma membrane lipid raft integrity and optimal T cell receptor (TCR) signaling. Moreover, UGCG-deficient CD8+ T cells display reduced granzyme expression, cytolytic activity, and tumor control in vivo. Together, our data establish GSL biosynthesis as a critical metabolic fate of glucose—beyond energy production—that is required for CD8+ T cell responses in vivo.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


