2-乙基己基取代环戊二噻吩的立体异构体分辨率及其对供体-受体-供体共轭分子光学性质的影响。
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
含两个2-乙基己基链的单甲酰化环戊二噻吩(CPDT-CHO)被成功地分解成三个立体异构体,分别为(R,S), (S,R)和(R,R)和(S,S)的混合物。不同的立体化学性质影响了相应的cpdt -受体- cpdt共轭分子的吸收光谱。本文章由计算机程序翻译,如有差异,请以英文原文为准。
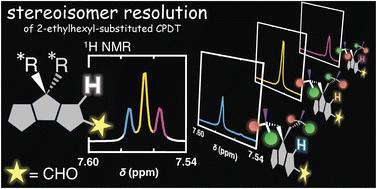
Stereoisomer resolution of 2-ethylhexyl-substituted cyclopentadithiophene and its effect on optical properties of donor–acceptor–donor conjugated molecules
Mono-formylated cyclopentadithiophene bearing two 2-ethylhexyl chains (CPDT-CHO) was successfully resolved into three stereoisomer fractions, assigned as (R,S), (S,R), and a mixture of (R,R) and (S,S). The distinct stereochemistry influenced the absorption spectra of the corresponding CPDT–acceptor–CPDT conjugated molecules.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


