合成α-羰基胺的稳定亚胺替代物。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报道了一种通过两种不同途径使用稳定的亚胺替代物合成α-羰基胺的通用方法。该方案在温和的条件下(室温)操作,提供高分离收率(高达99%),表现出优异的选择性(主要是z构型),并适应广泛的底物范围,包括功能化胺和羰基化合物。该方法解决了传统方法的局限性,如中间体不稳定和反应条件苛刻,为胺类药物和杂环化合物的制备提供了一条实用的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
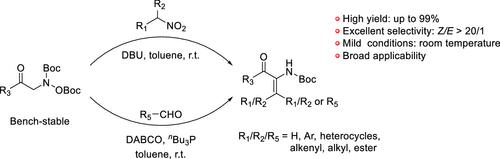
Bench-Stable Imine Surrogates for the Synthesis of α-Carbonylenamines
We report a versatile methodology for the synthesis of α-carbonylenamines using bench-stable imine surrogates via two distinct pathways. The protocol operates under mild conditions (room temperature), delivers high isolated yields (up to 99%), exhibits excellent selectivity (predominantly Z-configuration), and accommodates a broad substrate scope including functionalized amines and carbonyl compounds. This approach addresses limitations of traditional methods, such as instability of intermediates and harsh reaction conditions, and offers a practical route to enamine-based pharmaceuticals and heterocycles.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


