重新设计的弹性蛋白结构域衍生蛋白继承天然人类弹性蛋白特性。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
弹性蛋白的特点是其特殊的弹性和耐久性,抗降解,延长寿命,并能与细胞相互作用。这些有利的特性推动了对弹性蛋白样多肽(ELPs)的广泛研究。尽管ELPs表现出令人满意的特性,但它们无法完全包含人类弹性蛋白的复杂性,这是一个显著的局限性。因此,利用人类对角弹性蛋白的特定片段设计了专门的工程多肽,以创建适合于组织工程的生物相容性弹性蛋白样生物材料。在这项研究中,我们重新设计并构建了三种不同类型的弹性蛋白结构域衍生蛋白(eddp),每种都含有疏水、交联和细胞相互作用结构域,每种聚合物中重复结构域的数量各不相同。在细菌表达系统中表达重组EDDPs后,我们研究了它们的力学性能,包括弹性模量。重新设计的eddp具有良好的机械性能,生物相容性和细胞相互作用能力,使其适合作为生物材料。这些发现突出了重新设计的eddp在各种组织工程和再生医学应用中的潜力。意义说明:弹性蛋白是人体必需的蛋白质,在维持各种结缔组织的功能和结构中起着至关重要的作用。尽管弹性蛋白样多肽(ELPs)表现出理想的特性,但它们无法完全包含人类弹性蛋白的复杂性,这是一个显著的局限性。在这项研究中,我们旨在设计弹性蛋白样生物材料,在克服天然和传统弹性蛋白的局限性的同时,保留人类弹性蛋白的有利特性。我们相信我们的研究对生物材料领域做出了重大贡献,因为我们创造了三种重组弹性蛋白结构域衍生蛋白(eddp),克服了对偶弹性蛋白和ELP系统的局限性,同时保持了基本的弹性蛋白样特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
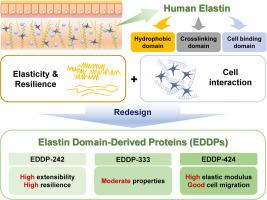
Redesigned elastin domain-derived proteins inherit natural human elastin properties
Elastin is distinguished by its exceptional elasticity and durability, resistance to degradation, prolonged lifespan, and ability to interact with cells. These favorable attributes have driven extensive research on elastin-like polypeptides (ELPs). Although ELPs exhibit desirable characteristics, their inability to fully encompass the intricacies of human elastin presents a notable limitation. Therefore, specifically engineered polypeptides have been designed using specific segments of human tropoelastin to create biocompatible elastin-like biomaterials suitable for tissue engineering. In this study, we redesigned and constructed three distinct types of elastin domain-derived proteins (EDDPs), each containing hydrophobic, cross-linking, and cellular interaction domains, with variations in the number of repeat domains within each polymer. Following the expression of recombinant EDDPs in a bacterial expression system, we investigated their mechanical properties, including the elastic modulus. The redesigned EDDPs exhibited favorable mechanical properties, biocompatibility, and cell-interaction capabilities, making them suitable as biomaterials. These findings highlight the potential of the redesigned EDDPs for various tissue engineering and regenerative medicine applications.
Statement of significance
Elastin, an essential protein in the human body, plays a crucial role in maintaining the functionality and structure of various connective tissues. Although elastin-like polypeptides (ELPs) exhibit desirable characteristics, their inability to fully encompass the intricacies of human elastin presents a notable limitation. In this study, we aimed to design elastin-like protein biomaterials that retain the favorable characteristics of human elastin while overcoming the limitations of natural and conventional ELPs. We believe that our study makes a significant contribution to the biomaterial field because we created three recombinant elastin domain-derived proteins (EDDPs) that overcome the limitations of the tropoelastin and ELP systems while maintaining essential elastin-like properties.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


