Ronaldo Aparecido da Silva, Renata Nakata Teixeira, Gerson Dos Santos Leite, Rosana C Agondi, Renata Gorjão, Cristina Maria Kokron, Yara Nely Fiks, Celso Ricardo Fernandes Carvalho
下载PDF
{"title":"药物治疗对耐力运动员运动性支气管收缩和过敏性炎症反应的影响。","authors":"Ronaldo Aparecido da Silva, Renata Nakata Teixeira, Gerson Dos Santos Leite, Rosana C Agondi, Renata Gorjão, Cristina Maria Kokron, Yara Nely Fiks, Celso Ricardo Fernandes Carvalho","doi":"10.26603/001c.141859","DOIUrl":null,"url":null,"abstract":"<p><strong>Background: </strong>Endurance athletes (EA) with lung disease and allergic inflammation have worse performance.</p><p><strong>Purpose: </strong>To examine whether pharmacological treatment can reduce airway disorders such as exercise-induced bronchoconstriction (EIB) and allergic inflammatory response (AIR) in EA.</p><p><strong>Study design: </strong>Prospective, controlled clinical trial.</p><p><strong>Methods: </strong>EA who were marathon runners underwent eucapnic voluntary hyperventilation (EVH) for screening assessment. EA who fulfilled the criteria for an EIB+ after an EVH were included in the treatment group (EIB+; n=13), and those who did not were included in the control group (CON; n=18). The athletes were assessed before and 30 days after the intervention. Outcomes included cardiopulmonary exercise testing, lung function, allergic symptoms (allergic questionnaire for athletes [AQUA©]), AIR (T helper [Th]-1, Th2, and Th17 lymphocytes in cell cultures), inflammatory mediator expression, salivary immunoglobulin (Ig)A, blood cortisol, blood IgE levels, and airway inflammation (fraction exhaled nitric oxide [FeNO]). Both groups were advised to keep the same training routine, and only the EIB+ received pharmacological treatment with inhaled corticosteroids (400-800 mcg/day) and long-acting bronchodilators (12 mcg/day). The CON and EIB+ groups underwent the same assessments after the intervention and were compared pre- and post-intervention, and effect sizes were calculated.</p><p><strong>Results: </strong>EIB+ (males, age 28.1±7.4 years, BMI 20.3±1.0 kg/m2) CON (males, age 29.8±6.5 years, BMI 20.5±1.6 kg/m2) participated. At baseline, the O2 peak, lung function, allergic symptoms, IgE, IgA, FeNO levels, and AIR were not significancly different between groups (p>0.05). After pharmacological treatment, only the EIB+ group showed a decrease in EIB (p<0.001) and an increase in VO2peak compared to baseline (p<0.05). However, no difference was observed in the expression of inflammatory mediators (p>0.05).</p><p><strong>Conclusion: </strong>Pharmacological treatment reduces EIB and increases the aerobic perforance/fitness in endurance athletes. These benefits occur without modification of the AIR.</p><p><strong>Level of evidence: </strong>Level II- Prospective Comparative Study.</p>","PeriodicalId":47892,"journal":{"name":"International Journal of Sports Physical Therapy","volume":"20 8","pages":"1222-1231"},"PeriodicalIF":2.1000,"publicationDate":"2025-08-01","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12317798/pdf/","citationCount":"0","resultStr":"{\"title\":\"Effect of Pharmacological Treatment on Exercise-Induced Bronchoconstriction and Allergic Inflammatory Response in Endurance Athletes.\",\"authors\":\"Ronaldo Aparecido da Silva, Renata Nakata Teixeira, Gerson Dos Santos Leite, Rosana C Agondi, Renata Gorjão, Cristina Maria Kokron, Yara Nely Fiks, Celso Ricardo Fernandes Carvalho\",\"doi\":\"10.26603/001c.141859\",\"DOIUrl\":null,\"url\":null,\"abstract\":\"<p><strong>Background: </strong>Endurance athletes (EA) with lung disease and allergic inflammation have worse performance.</p><p><strong>Purpose: </strong>To examine whether pharmacological treatment can reduce airway disorders such as exercise-induced bronchoconstriction (EIB) and allergic inflammatory response (AIR) in EA.</p><p><strong>Study design: </strong>Prospective, controlled clinical trial.</p><p><strong>Methods: </strong>EA who were marathon runners underwent eucapnic voluntary hyperventilation (EVH) for screening assessment. EA who fulfilled the criteria for an EIB+ after an EVH were included in the treatment group (EIB+; n=13), and those who did not were included in the control group (CON; n=18). The athletes were assessed before and 30 days after the intervention. Outcomes included cardiopulmonary exercise testing, lung function, allergic symptoms (allergic questionnaire for athletes [AQUA©]), AIR (T helper [Th]-1, Th2, and Th17 lymphocytes in cell cultures), inflammatory mediator expression, salivary immunoglobulin (Ig)A, blood cortisol, blood IgE levels, and airway inflammation (fraction exhaled nitric oxide [FeNO]). Both groups were advised to keep the same training routine, and only the EIB+ received pharmacological treatment with inhaled corticosteroids (400-800 mcg/day) and long-acting bronchodilators (12 mcg/day). The CON and EIB+ groups underwent the same assessments after the intervention and were compared pre- and post-intervention, and effect sizes were calculated.</p><p><strong>Results: </strong>EIB+ (males, age 28.1±7.4 years, BMI 20.3±1.0 kg/m2) CON (males, age 29.8±6.5 years, BMI 20.5±1.6 kg/m2) participated. At baseline, the O2 peak, lung function, allergic symptoms, IgE, IgA, FeNO levels, and AIR were not significancly different between groups (p>0.05). After pharmacological treatment, only the EIB+ group showed a decrease in EIB (p<0.001) and an increase in VO2peak compared to baseline (p<0.05). However, no difference was observed in the expression of inflammatory mediators (p>0.05).</p><p><strong>Conclusion: </strong>Pharmacological treatment reduces EIB and increases the aerobic perforance/fitness in endurance athletes. These benefits occur without modification of the AIR.</p><p><strong>Level of evidence: </strong>Level II- Prospective Comparative Study.</p>\",\"PeriodicalId\":47892,\"journal\":{\"name\":\"International Journal of Sports Physical Therapy\",\"volume\":\"20 8\",\"pages\":\"1222-1231\"},\"PeriodicalIF\":2.1000,\"publicationDate\":\"2025-08-01\",\"publicationTypes\":\"Journal Article\",\"fieldsOfStudy\":null,\"isOpenAccess\":false,\"openAccessPdf\":\"https://www.ncbi.nlm.nih.gov/pmc/articles/PMC12317798/pdf/\",\"citationCount\":\"0\",\"resultStr\":null,\"platform\":\"Semanticscholar\",\"paperid\":null,\"PeriodicalName\":\"International Journal of Sports Physical Therapy\",\"FirstCategoryId\":\"1085\",\"ListUrlMain\":\"https://doi.org/10.26603/001c.141859\",\"RegionNum\":0,\"RegionCategory\":null,\"ArticlePicture\":[],\"TitleCN\":null,\"AbstractTextCN\":null,\"PMCID\":null,\"EPubDate\":\"2025/1/1 0:00:00\",\"PubModel\":\"eCollection\",\"JCR\":\"Q3\",\"JCRName\":\"SPORT SCIENCES\",\"Score\":null,\"Total\":0}","platform":"Semanticscholar","paperid":null,"PeriodicalName":"International Journal of Sports Physical Therapy","FirstCategoryId":"1085","ListUrlMain":"https://doi.org/10.26603/001c.141859","RegionNum":0,"RegionCategory":null,"ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"2025/1/1 0:00:00","PubModel":"eCollection","JCR":"Q3","JCRName":"SPORT SCIENCES","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Effect of Pharmacological Treatment on Exercise-Induced Bronchoconstriction and Allergic Inflammatory Response in Endurance Athletes.
Background: Endurance athletes (EA) with lung disease and allergic inflammation have worse performance.
Purpose: To examine whether pharmacological treatment can reduce airway disorders such as exercise-induced bronchoconstriction (EIB) and allergic inflammatory response (AIR) in EA.
Study design: Prospective, controlled clinical trial.
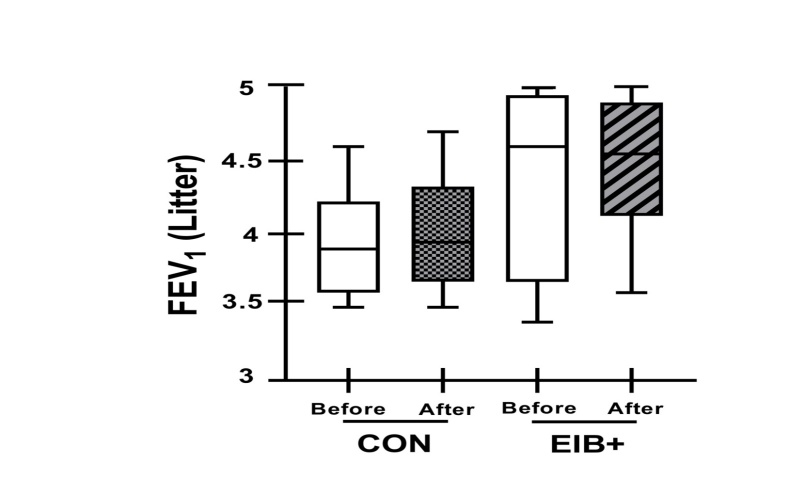
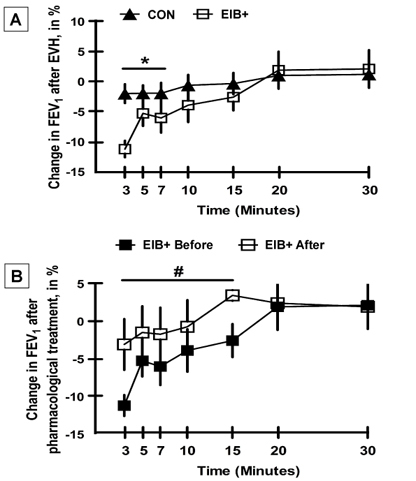
Methods: EA who were marathon runners underwent eucapnic voluntary hyperventilation (EVH) for screening assessment. EA who fulfilled the criteria for an EIB+ after an EVH were included in the treatment group (EIB+; n=13), and those who did not were included in the control group (CON; n=18). The athletes were assessed before and 30 days after the intervention. Outcomes included cardiopulmonary exercise testing, lung function, allergic symptoms (allergic questionnaire for athletes [AQUA©]), AIR (T helper [Th]-1, Th2, and Th17 lymphocytes in cell cultures), inflammatory mediator expression, salivary immunoglobulin (Ig)A, blood cortisol, blood IgE levels, and airway inflammation (fraction exhaled nitric oxide [FeNO]). Both groups were advised to keep the same training routine, and only the EIB+ received pharmacological treatment with inhaled corticosteroids (400-800 mcg/day) and long-acting bronchodilators (12 mcg/day). The CON and EIB+ groups underwent the same assessments after the intervention and were compared pre- and post-intervention, and effect sizes were calculated.
Results: EIB+ (males, age 28.1±7.4 years, BMI 20.3±1.0 kg/m2) CON (males, age 29.8±6.5 years, BMI 20.5±1.6 kg/m2) participated. At baseline, the O2 peak, lung function, allergic symptoms, IgE, IgA, FeNO levels, and AIR were not significancly different between groups (p>0.05). After pharmacological treatment, only the EIB+ group showed a decrease in EIB (p<0.001) and an increase in VO2peak compared to baseline (p<0.05). However, no difference was observed in the expression of inflammatory mediators (p>0.05).
Conclusion: Pharmacological treatment reduces EIB and increases the aerobic perforance/fitness in endurance athletes. These benefits occur without modification of the AIR.
Level of evidence: Level II- Prospective Comparative Study.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


