抗菌防腐剂对单克隆抗体曲妥珠单抗蛋白折叠稳定性和亚可见颗粒形成的影响。
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2025-08-05
DOI:10.1016/j.ejpb.2025.114835
引用次数: 0
摘要
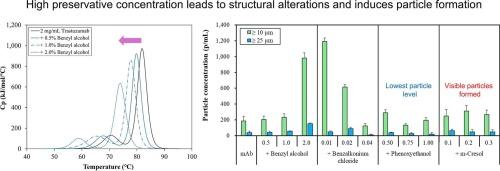
为了防止微生物污染,需要在多剂量生物药品中添加抗菌防腐剂;然而,它通常会给蛋白质稳定性带来风险,潜在地损害治疗效果。在这项研究中,我们研究了不同防腐剂(苯甲醇、间甲酚、苯氧乙醇和苯扎氯铵)对曲妥珠单抗生物物理稳定性的影响。曲妥珠单抗是一种广泛用于治疗HER2受体阳性癌症的单克隆抗体。经5 天的搅拌后,苯甲醇(1.0 % v/v)和间甲酚(0.3 % w/v)显著降低了单体含量。苯甲醇与纳米到微型颗粒的激增(增加21倍)和热稳定性降低(ΔTm: -5.39 °C)有关。m-甲酚在72 h内触发可见颗粒形成(bbb100 µm),引起对注射生物制剂的关注。苯扎氯铵(0.01 %-0.04 % w/v)表现出不一致的浓度依赖行为,最初表现出亚可见聚集体的增加,然后在更高浓度下通过胶束形成稳定,尽管二级结构向β-片基序不可逆地转移。相反,苯氧乙醇(0.5 % v/v)表现出更高的相容性,保持了单体含量,并将颗粒生成抑制到基线水平。这些发现强调了在治疗性生物制剂的配方设计中进行防腐剂特异性相容性评估的必要性,将苯氧乙醇定位为曲妥珠单抗保存的有希望的候选者,因为它在抗菌功效和最小不稳定性之间取得了平衡。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Effects of antimicrobial preservatives on protein folding stability and subvisible particle formation in monoclonal antibody trastuzumab
To prevent microbial contamination, antimicrobial preservatives need to be added in multi-dose biopharmaceuticals; however, it often introduces risks to protein stability, potentially compromising therapeutic efficacy. In this study, we investigated the effects of different preservatives (benzyl alcohol, m-cresol, phenoxyethanol, and benzalkonium chloride) on the biophysical stability of trastuzumab, a monoclonal antibody widely used for treatment of HER2 receptor-positive cancers. Among the preservatives tested, benzyl alcohol (1.0 % v/v) and m-cresol (0.3 % w/v) significantly reduced the monomeric content after 5 days of end-over-end agitation stress. Benzyl alcohol was associated with a surge in nano- to micro-sized particles (21-fold increase) and decreased thermal stability (ΔTm: −5.39 °C). m-Cresol uniquely triggered visible particle formation (>100 µm) within 72 h, raising concerns for injectable biologics. Benzalkonium chloride (0.01 %–0.04 % w/v) exhibited inconsistent concentration-dependent behavior, initially showing increase in subvisible aggregates before stabilizing through micelle formation at higher concentrations, albeit with irreversible secondary structural shifts toward β-sheet motifs. Conversely, phenoxyethanol (0.5 % v/v) exhibited higher compatibility, preserved the monomeric content, and suppressed particle generation to baseline levels. These findings underscore the necessity of preservative-specific compatibility assessments in formulation design for therapeutic biologics, positioning phenoxyethanol as a promising candidate for trastuzumab preservation owing to the balance between its antimicrobial efficacy and minimal destabilization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


