利用液-液相分离技术探索丝蛋白的自组装
IF 2.7
4区 化学
Q3 POLYMER SCIENCE
引用次数: 0
摘要
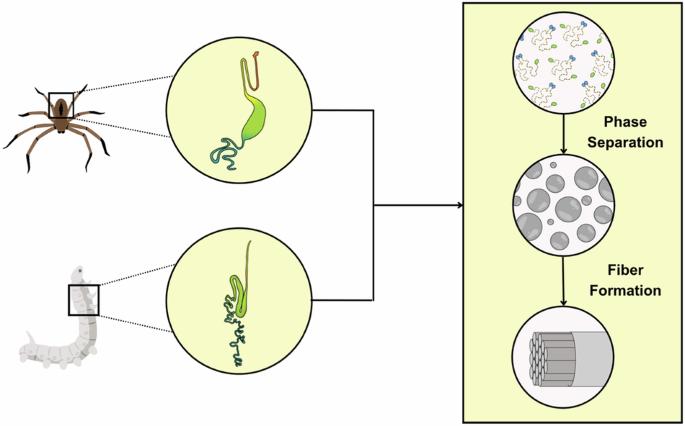
人类几千年来一直使用丝绸纤维来制造纺织品,最近由于其无与伦比的机械性能而引起了科学家的注意。这些特性来自于一个复杂的过程,通过这个过程,原料(一种由高分子量丝蛋白组成的液体原料)在丝腺内迅速转化为具有多尺度分层结构的固体纤维,这是材料令人难以置信的坚固性的原因。近年来,液-液相分离(LLPS)已成为理解丝蛋白自组装行为的有力框架。有趣的是,llps相关蛋白通常表现出无序或动态构象,并且具有丰富的低复杂性多价重复序列,让人想起丝绸蛋白序列。在这篇综述中,我们探讨了证明LLPS是鳞翅目和蜘蛛系统中丝纤维储存和组装的主要方面的证据。我们讨论了从氨基酸序列、特定化学触发因素和潜在化学相互作用的比较分析中获得的见解,并将最近基于天然和重组丝绸材料的实证研究结果背景化。我们还讨论了如何将LLPS机制应用于复制原生分层结构的丝状材料的可持续生产。最后,我们概述了未来研究的重要领域,并推测丝绸研究领域的发现如何有助于阐明生物分子凝聚物的更广泛领域。蜘蛛和家蚕的产丝过程包括将浓缩的液体蛋白质原料转化为有层次组织的固体纤维,这是由它们各自的腺体纺丝装置高度控制的机制促成的。最近的见解表明,液-液相分离(LLPS)在将最初无序的丝蛋白链组织成致密但动态的凝聚体中起着核心作用,这是快速形成纤维的关键一步。这种分层组装过程奠定了丝纤维卓越的机械性能。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Exploring the self-assembly of silk proteins through liquid-liquid phase separation
Silk fibers have been used by humans for millennia to create textiles and have recently gained the attention of scientists due to their unsurpassed mechanical properties. These properties arise from a sophisticated process by which the starting material, a liquid feedstock consisting of high-molecular-weight silk proteins, is rapidly converted within silk glands into solid fibers with a multi-scale hierarchical structure that is responsible for the material’s incredible robustness. Recently, liquid-liquid phase separation (LLPS) has emerged as a powerful framework for understanding the self-assembly behavior of silk proteins. Interestingly, LLPS-associated proteins typically exhibit disordered or dynamic conformations and have sequences rich in low-complexity multivalent repeats, reminiscent of silk protein sequences. In this review, we explore the evidence indicating that LLPS is a major aspect of silk fiber storage and assembly in both lepidopteran and spider systems. We discuss insights derived from comparative analyses of amino acid sequences, specific chemical triggers, and potential chemical interactions and contextualize the results from recent empirical investigations based on native and recombinant silk materials. We also discuss how LLPS mechanisms might be applied to the sustainable production of silk-like materials that replicate native hierarchical structures. Finally, we outline important areas for future investigations and speculate on how findings from the field of silk research may help illuminate the more general field of biomolecular condensates. The production of silk in spiders and silkworms involves the transformation of concentrated liquid protein feedstock into hierarchically organized solid fibers through a highly controlled mechanism facilitated by their respective glandular spinning apparatus. Recent insights suggest that liquid–liquid phase separation (LLPS) plays a central role in organizing the initially disordered silk protein chains into dense yet dynamic condensates, which is a key step towards rapid fiber formation. This hierarchical assembly process underlies the remarkable mechanical properties of silk fibers.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Polymer Journal
化学-高分子科学
CiteScore
5.60
自引率
7.10%
发文量
131
审稿时长
2.5 months
期刊介绍:
Polymer Journal promotes research from all aspects of polymer science from anywhere in the world and aims to provide an integrated platform for scientific communication that assists the advancement of polymer science and related fields. The journal publishes Original Articles, Notes, Short Communications and Reviews.
Subject areas and topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Polymer synthesis and reactions
Polymer structures
Physical properties of polymers
Polymer surface and interfaces
Functional polymers
Supramolecular polymers
Self-assembled materials
Biopolymers and bio-related polymer materials
Polymer engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


