Sn-Sb-Ce/GAC颗粒电极对高盐废水中甲基异噻唑啉酮的强化去除:反应种类和效率
IF 8.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
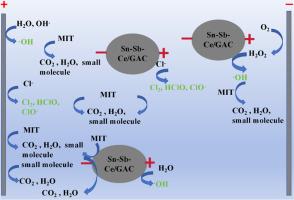
深度氧化法是降解工业废水中化学稳定性高、难降解的甲基异噻唑啉酮类杀菌剂的有效方法。为了组装一种低成本、高性能的废水处理电化学氧化系统,采用溶胶-凝胶法在颗粒活性炭(GAC)上掺杂Ce、Sn、Sb修饰,作为颗粒电极填充到三维(3D)电化学反应器中,合成Sn-Sb-Ce/GAC。扫描电镜(SEM)、能谱(EDS)和x射线衍射(XRD)实验表明,Sn-Sb-Ce/GAC颗粒电极晶体颗粒致密均匀,表面结构得到改善。十次循环实验表明,Sn-Sb-Ce/GAC颗粒电极稳定性高,负载活性物质溶出率低。在GAC为5 g/L、电流密度为20 mA/cm2、初始pH为5、电解液Na2SO4浓度为0.02 mol/L、反应时间为120 min的条件下,研究了MIT的降解机理。MIT的间接电化学降解以活性物质途径为主,活性氯而非自由基(•OH)起主要作用。与传统的二维(2D)电极系统相比,三维电化学系统具有更大的活性电极面积、更高的处理效率和更低的能耗。三维电化学系统在30 min的时间内,可从实际高盐反渗透浓缩废水中去除96.5 %的MIT。对废水中的UV254有一定的去除效果,但对荧光物质的去除效果更好。本研究提出了利用碳基材料开发过渡金属和稀土金属颗粒电极在电化学处理系统中实现高效电催化氧化的新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhanced removal of methylisothiazolinone from high-salt wastewater by Sn-Sb-Ce/GAC particle electrode: Reactive species and efficiency
Advanced oxidation processes are promising for degradation of the highly chemical stability and refractory methylisothiazolinone (MIT) bactericides in relevant industrial wastewater. In order to assemble a low cost and high performance electrochemical oxidation system for wastewater treatment, granular active carbon (GAC) was decorated by doping Ce, Sn, Sb to synthesize Sn-Sb-Ce/GAC using sol-gel method as particle electrode filled into a three-dimensional (3D) electrochemical reactor. Scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDS) and X-ray diffraction (XRD) experiments revealed that the Sn-Sb-Ce/GAC particle electrode crystal particles were compact and uniform, and the surface structure was improved. The ten cyclic experiments indicated that the Sn-Sb-Ce/GAC particle electrode had high stability and low dissolution of the loaded active substance. The degradation mechanism of MIT was studied under the optimal working conditions of 3D electrode system with GAC of 5 g/L, current density of 20 mA/cm2, initial pH 5, electrolyte concentration of Na2SO4 0.02 mol/L and reaction time of 120 min. The indirect electrochemical degradation of MIT was dominated by active substance pathway that active chlorine rather than free radicals (•OH) played the main role. Comparing with conventional two-dimensional (2D) electrode system, the 3D electrochemical system has larger active electrode area, higher treatment efficiency and lower energy consumption than the former. The 3D electrochemical system could remove 96.5 % of MIT from the actual high-salt reverse osmosis concentrate wastewater in 30 min. It has a certain removal effect on UV254 in wastewater, but has a better removal effect on fluorescent substances. This study proposed a new strategy to develop transition metal and rare earth metal particle electrodes using carbon-based materials for high efficient electrocatalytic oxidation in the electrochemical treatment system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


