用于再生软组织修复的仿生丝素网:一种时空活性支架的多尺度评价
IF 6
2区 医学
Q2 MATERIALS SCIENCE, BIOMATERIALS
Materials Science & Engineering C-Materials for Biological Applications
Pub Date : 2025-07-31
DOI:10.1016/j.bioadv.2025.214435
引用次数: 0
摘要
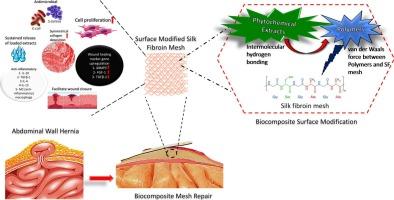
为了解决传统/商业手术补片相关的并发症,如纤维化、血肿和细菌感染,本研究强调了使用轻质、大孔、全天然材料补片用于腹壁愈合、软组织修复和再生的好处。方法首次将丝素网与自旋辅助浸渍生物聚合物-植物化学复合材料结合,用于软组织修复。用自旋助浸生物聚合物-植物化学涂层对经纬手织丝素(SF)网进行表面功能化。5%天然提取物(LE和BE)替代抗生素,具有抗菌、抗氧化和抗炎作用,且无毒。量身定制的12% PHBV、PLA和PCL涂层能够控制药物释放和延长降解时间,并与组织修复相一致。对这些改进的网格进行了四个阶段的分析,即材料表征,计算机评估,体外测试和体内分析。结果PHBV修饰后的网状结构孔径减小,纤维直径增大(分别为623.9±66.7 μm和12.1±2.9 μm),是一种有效的药物传递系统。其中PHBV-LE为最有效的复合材料,其释放动力学为70.4%,表明LE的中间释放介导了有效的抗菌特性。PHBV-LE变异体具有促进成纤维细胞增殖(121.5%)、有效的细胞附着、伤口愈合(88.27%)和关键伤口愈合标志物(MMP3、FGF-1、TGFβ-1)基因显著上调的优势。在大鼠模型(Dawley)的体内分析显示,PHBV-LE加速了组织整合和胶原沉积,表明PHBV-LE有效地修复和再生组织。结论该材料具有优良的材料性能、抗菌性能和创面愈合性能,具有结构轻、孔径优、给药效率高等优点。在所有仿生SF复合材料中,PHBV-LE模拟了天然细胞外基质的优越特性,可作为软组织修复和体外治疗评估的多功能平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Biomimetic silk fibroin meshes for regenerative soft tissue repair: Multiscale evaluation of a spatiotemporally active scaffold
Background
To address traditional/commercial surgical meshes linked complications like fibrosis, seroma, and bacterial infections, this study highlights the benefits of using lightweight, large-pore, all natural material meshes for abdominal wall healing, soft tissue repair and regeneration.
Method
This study presents a first-of-its-kind approach combining hand-knitted silk fibroin (SF) meshes with spin-assisted dip-coated biopolymer–phytochemical composites for soft tissue repair. The multifunctional mesh fabricated via a sustainable crochet method- weft hand-knitted silk fibroin (SF) meshes was surface-functionalized with spin-assisted dip biopolymer–phytochemical coatings. 5 % natural extracts (LE and BE) replace antibiotics, providing antimicrobial, antioxidant, and anti-inflammatory effects without toxicity. Tailored 12 % PHBV, PLA, and PCL coatings enable controlled drug release and extended degradation aligned with tissue repair. These modified meshes were analyzed in four phases i.e. material characterization, in silico assessments, in vitro testing, and in vivo analysis.
Results
Among the variants, PHBV modified meshes as an efficient drug delivery system with reduced pore size and increased fiber diameter (623.9 ± 66.7 μm and 12.1 ± 2.9 μm respectively). Specifically PHBV-LE emerged as the most effective composite with release kinetics of 70.4 % showing intermediate release of LE mediating the potent antimicrobial character. The PHBV-LE variant was a superior candidate with pore size promoting fibroblast proliferation (121.5 %), effective cell attachment, wound closure (88.27 %), and highly significant gene upregulation of key wound-healing markers (MMP3, FGF-1, TGFβ-1). In vivo analysis in rat models (Dawley) showed accelerated tissue integration and collagen deposition, indicating effective tissue repair and regeneration by PHBV-LE.
Conclusion
These meshes exhibited excellent material, antibacterial, and wound-healing properties, with lightweight structure, optimal pore size, and efficient drug delivery. Among all biomimetic SF composites, PHBV-LE emulates superior native extracellular matrix features, serving as multifunctional platforms for soft tissue repair and in vitro therapeutic evaluation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
17.80
自引率
0.00%
发文量
501
审稿时长
27 days
期刊介绍:
Biomaterials Advances, previously known as Materials Science and Engineering: C-Materials for Biological Applications (P-ISSN: 0928-4931, E-ISSN: 1873-0191). Includes topics at the interface of the biomedical sciences and materials engineering. These topics include:
• Bioinspired and biomimetic materials for medical applications
• Materials of biological origin for medical applications
• Materials for "active" medical applications
• Self-assembling and self-healing materials for medical applications
• "Smart" (i.e., stimulus-response) materials for medical applications
• Ceramic, metallic, polymeric, and composite materials for medical applications
• Materials for in vivo sensing
• Materials for in vivo imaging
• Materials for delivery of pharmacologic agents and vaccines
• Novel approaches for characterizing and modeling materials for medical applications
Manuscripts on biological topics without a materials science component, or manuscripts on materials science without biological applications, will not be considered for publication in Materials Science and Engineering C. New submissions are first assessed for language, scope and originality (plagiarism check) and can be desk rejected before review if they need English language improvements, are out of scope or present excessive duplication with published sources.
Biomaterials Advances sits within Elsevier''s biomaterials science portfolio alongside Biomaterials, Materials Today Bio and Biomaterials and Biosystems. As part of the broader Materials Today family, Biomaterials Advances offers authors rigorous peer review, rapid decisions, and high visibility. We look forward to receiving your submissions!

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


