心理质体原tabernanthalog诱导神经可塑性,但没有直接的早期基因激活
IF 20
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
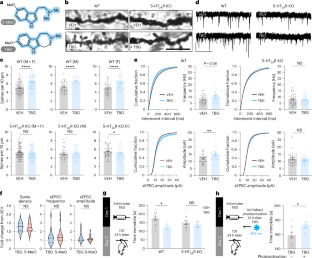
非致幻性精神致癌剂,如他伯南他洛(TBG),正在被开发为可能更安全、更可扩展的致幻剂替代品,用于促进神经元生长和治疗各种脑部疾病。目前,尚不清楚5-羟色胺2A (5-HT2A)受体和即时早期基因(IEG)激活是否在非致幻性精神致塑剂的神经可塑性促进作用中起作用。在这里,我们使用药理学和遗传学工具在啮齿类动物中表明,非致幻性精神塑原通过与经典致幻剂相同的生化途径(包括5-HT2A、TrkB、mTOR和AMPA受体激活)促进皮质神经可塑性,并且TBG诱导的皮质脊髓发生是TBG持续抗抑郁样行为效果所必需的。与致幻剂相比,TBG不会立即引起谷氨酸爆发或IEG激活。由于这些作用被认为是致幻剂诱导的神经可塑性的必要条件,我们的研究结果揭示了某些精神致幻剂在没有致幻剂作用的情况下促进皮层神经可塑性的机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

The psychoplastogen tabernanthalog induces neuroplasticity without proximate immediate early gene activation
Nonhallucinogenic psychoplastogens, such as tabernanthalog (TBG), are being developed as potentially safer, more scalable alternatives to psychedelics for promoting neuronal growth and treating various brain conditions. Currently, it is unclear whether 5-hydroxytryptamine 2A (5-HT2A) receptors and immediate early gene (IEG) activation have a role in the neuroplasticity-promoting effects of nonhallucinogenic psychoplastogens. Here, we use pharmacological and genetic tools in rodents to show that nonhallucinogenic psychoplastogens promote cortical neuroplasticity through the same biochemical pathway—involving 5-HT2A, TrkB, mTOR and AMPA receptor activation—as classic psychedelics and that TBG-induced cortical spinogenesis is required for the sustained antidepressant-like behavioral effect of TBG. In contrast to psychedelics, TBG does not induce an immediate glutamate burst or IEG activation. As these effects have been assumed to be necessary for psychedelic-induced neuroplasticity, our results shed light on the mechanisms by which certain psychoplastogens can promote cortical neuroplasticity in the absence of hallucinogenic effects. Aarrestad et al. show that, in contrast to psychedelics, the nonhallucinogenic psychoplastogen tabernanthalog promotes neuroplasticity through 5-HT2AR activation without immediately increasing extracellular glutamate levels or immediate early gene expression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


