利用基因组结构方程模型揭示脆弱性的多变量遗传结构
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
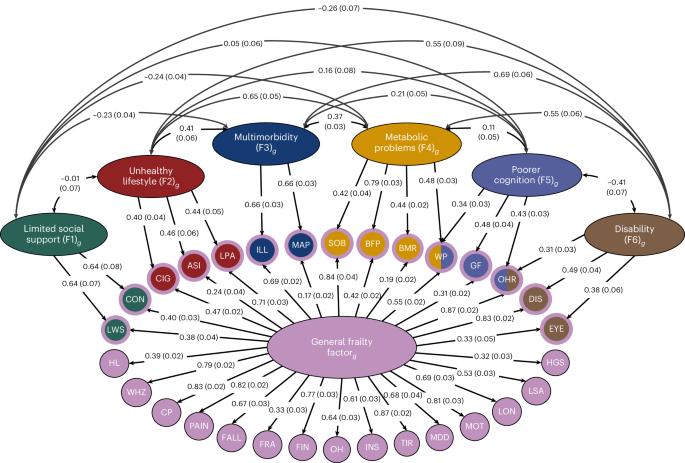
虚弱是一种多方面的临床状态,与加速衰老和不良健康结果有关。知情的虚弱病因学模型有望在老龄人口中产生广泛的健康改善。虚弱目前是用总分来衡量的,这模糊了只与虚弱的子成分相关的病因途径。在这里,我们对30个脆弱缺陷之间的潜在遗传结构进行了多变量全基因组关联研究,确定了408个基因组风险位点。我们的模型包括所有缺陷的遗传重叠的一般因素,加上六个新的因素,索引特定缺陷群体的共享遗传信号。我们展示了这六个因素的附加临床和病因学价值,包括预测外部数据集的脆弱性,强调与临床相关结果的不同遗传相关性,以及揭示与衰老相关的独特潜在生物学。我们表明,细致入微的虚弱模型是理解其原因及其与健康状况恶化之间关系的关键。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Uncovering the multivariate genetic architecture of frailty with genomic structural equation modeling
Frailty is a multifaceted clinical state associated with accelerated aging and adverse health outcomes. Informed etiological models of frailty hold promise for producing widespread health improvements across the aging population. Frailty is currently measured using aggregate scores, which obscure etiological pathways that are only relevant to subcomponents of frailty. Here we perform a multivariate genome-wide association study of the latent genetic architecture between 30 frailty deficits, which identifies 408 genomic risk loci. Our model includes a general factor of genetic overlap across all deficits, plus six new factors indexing a shared genetic signal across specific groups of deficits. We demonstrate the added clinical and etiological value of the six factors, including predicting frailty in external datasets, highlighting divergent genetic correlations with clinically relevant outcomes and uncovering unique underlying biology linked to aging. We show that nuanced models of frailty are key to understanding its causes and how it relates to worse health. Multivariate genome-wide association analyses of the latent genetic architecture of frailty identify one general factor of genetic overlap across all frailty deficits and six factors indexing a shared genetic signal across specific groups of deficits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


