单一对虾卵巢特异性Kelch结构域基因的鉴定和功能表征。
IF 2.2
3区 生物学
Q4 BIOCHEMISTRY & MOLECULAR BIOLOGY
Comparative Biochemistry and Physiology A-Molecular & Integrative Physiology
Pub Date : 2025-07-31
DOI:10.1016/j.cbpa.2025.111914
引用次数: 0
摘要
在甲壳类动物中,卵黄蛋白原(Vg)的合成受复杂的激素和分子网络的调控。本研究探讨了蜕皮激素受体(PmEcR)在单对虾(Penaeus monodon)中调控Vg表达的作用,并鉴定了下游效应物。使用RNA干扰沉默PmEcR导致Vg表达增加两倍,性腺指数(gonadosomatic index, GSI)显著升高,表明PmEcR是卵黄形成的抑制因子。通过抑制减法杂交(SSH)技术,鉴定了一个未表征的LOC 113805388基因在PmEcR沉默时被下调。功能和结构表征表明,该转录物为卵巢特异性蛋白,具有β-螺旋桨结构(Kelch-like domain containing protein;这表明它属于kelch样蛋白家族,通常参与细胞调节中的蛋白质相互作用。卵巢蛋白质组的LC-MS/MS分析证实了PmKel蛋白的存在,证实了其在卵巢中的表达。在卵巢发育过程中,PmKel的表达与Vg的表达呈负相关,其敲低与Vg转录物水平升高有关,提示其可能参与了Vg基因表达的调控。此外,制备了重组PmKel蛋白,并利用亲和纯化质谱(AP-MS)作为诱饵,鉴定其在卵巢核提取物中的伴侣。分离的相互作用蛋白组突出了mRNA和参与发育途径的蛋白质代谢过程的功能富集。此外,通过蛋白网络分析和蛋白对接,这些蛋白可以与PmKel 3D蛋白结构中预测的Kelch-like结构域相互作用。这些发现表明PmKel是一种新的卵巢特异性含kelch蛋白,通过EcR途径在卵黄形成调节网络中起关键作用。总的来说,这些结果增加了对虾繁殖的现有知识,并可能为提高水产养殖的繁殖性能提供未来的方向。本文章由计算机程序翻译,如有差异,请以英文原文为准。
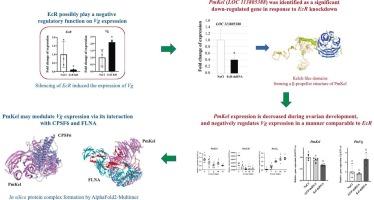
Identification and functional characterization of a novel ovarian-specific Kelch domain-containing gene involved in the ecdysteroid signaling pathway of Penaeus monodon
In crustaceans, vitellogenin (Vg) synthesis is regulated by complex hormonal and molecular networks. This study investigates the role of the ecdysone receptor (PmEcR) in regulating Vg expression and identifies downstream effectors in Penaeus monodon. Silencing of PmEcR using RNA interference resulted in a two-fold increase in Vg expression and a significant rise in the gonadosomatic index (GSI), suggesting PmEcR acts as a suppressor of vitellogenesis.
Through a suppression subtractive hybridization (SSH) technique, an uncharacterized LOC 113805388 gene, was identified as being downregulated upon PmEcR silencing. Functional and structural characterization revealed this transcript as an ovarian-specific protein with a β-propeller structure (Kelch-like domain-containing protein; PmKel), suggesting that it belongs to a Kelch-like protein family generally involved in protein-protein interactions in cellular regulation. LC-MS/MS analysis of the ovarian proteome confirmed the presence of the PmKel protein, verifying its expression in the ovary. PmKel expression displayed an inverse pattern to that of Vg during ovarian development, and its knockdown was associated with increased Vg transcript levels, suggesting a possible role in the regulation of Vg gene expression. In addition, a recombinant PmKel protein was produced and used as a bait to identify its partners in ovarian nuclear extract by affinity purification mass spectrometry (AP-MS). The isolated set of interacting proteins highlighted functional enrichment in mRNA and protein metabolic processes involved in developmental pathways. Also, these proteins could interact with a Kelch-like domain predicted on PmKel 3D protein structure by protein network analysis and protein-protein docking. These findings suggest that PmKel is a novel ovarian specific Kelch-containing protein that plays a key role in the vitellogenesis regulatory network via the EcR pathway. Collectively, these results add to the current knowledge of shrimp reproduction and may suggest future direction for improving reproductive performance in aquaculture.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.00
自引率
4.30%
发文量
155
审稿时长
3 months
期刊介绍:
Part A: Molecular & Integrative Physiology of Comparative Biochemistry and Physiology. This journal covers molecular, cellular, integrative, and ecological physiology. Topics include bioenergetics, circulation, development, excretion, ion regulation, endocrinology, neurobiology, nutrition, respiration, and thermal biology. Study on regulatory mechanisms at any level of organization such as signal transduction and cellular interaction and control of behavior are also published.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


