以铜为锚定的卟啉基金属有机框架高效电催化CO2还原为CH4†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
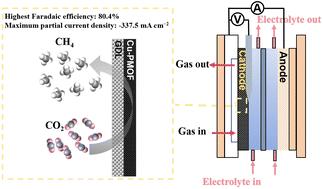
电催化二氧化碳(CO2)还原为甲烷(CH4)是一种很有前途的可持续碳循环策略。然而,在实际应用中,这种涉及8个电子转移过程的复杂转化在实现令人满意的催化活性和CH4选择性方面面临着重大挑战。本文采用简单的溶剂热反应策略,将铜(Cu)原子锚定在卟啉基金属有机骨架(PMOF)中,构建Cu单原子催化剂,命名为Cu-PMOF催化剂。Cu- pmof电极中高度可接近的Cu活性位点、优化的Cu负载和松散的结构的良好组合显著提高了CO2转化为CH4的电催化性能。当电流密度为−300 mA cm−2时,CH4的法拉第效率最高,达到80.4%。此外,与可逆氢电极相比,该Cu-PMOF电极在- 1.23 V电位下的最大分电流密度为- 337.5 mA cm - 2。综合实验研究表明,Cu-PMOF电极实现了多步CO2加氢过程,其特征是有效的H2O活化和CO2依次转化为关键中间体,最终导致CH4的选择性形成。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Porphyrin-based metal–organic frameworks anchored with Cu species for highly efficient electrocatalytic CO2 reduction to CH4†
The electrocatalytic carbon dioxide (CO2) reduction into methane (CH4) represents a promising strategy for sustainable carbon cycling. Nevertheless, this complex conversion, involving an eight-electron transfer process, faces significant challenges in achieving satisfactory catalytic activity and CH4 selectivity for practical applications. Herein, we employed a facile solvothermal reaction strategy to anchor copper (Cu) atoms in the porphyrin-based metal–organic framework (PMOF) to construct Cu single-atom catalysts, named the Cu-PMOF catalyst. The good combination of highly accessible Cu active sites, optimized Cu loading, and a loose structure in the Cu-PMOF electrode significantly enhanced the electrocatalytic performance for the conversion of CO2 to CH4. The highest Faradaic efficiency of CH4 reached 80.4% at a current density of −300 mA cm−2 in 1 M potassium hydroxide electrolytes with a flow cell configuration. Moreover, this Cu-PMOF electrode achieved a maximum partial current density of −337.5 mA cm−2 at a potential of −1.23 V versus reversible hydrogen electrode. Comprehensive experimental investigations revealed the Cu-PMOF electrode enabled a multi-step CO2 hydrogenation process, characterized by effective H2O activation and the sequential transformation of CO2 into crucial intermediates, ultimately leading to the selective formation of CH4.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


