氢化诱导PET废弃物的选择性降解研究
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
聚对苯二甲酸乙二醇酯(PET)是一种普遍存在的多用途热塑性聚合物,在日常生活中有着广泛的应用。然而,与其他合成塑料一样,PET的大规模生产和积累造成了沉重的环境负担。化学升级回收是解决PET塑料垃圾带来的环境危机的一种很有前途的策略,但其实际实施仍然具有挑战性。在此,我们报告了一种新的氢化诱导水热降解策略,用于PET的升级回收。首次在简单的中性水条件下,高效地将一系列高结晶PET消费后废弃物转化为高价值的1,4-环己二羧酸(1,4- chda)和乙二醇(EG)。详细的机理研究表明,相对于酯键的水解,PET链上芳香环的氢化优先发生。氢化过程不仅消除了分子间的π -π堆积力,而且破坏了分子内共轭π-体系,从而提高了PET中酯键的亲电性和水解反应性。这项工作为PET塑料的升级回收开辟了一条新的经济有效的途径,这可能会刺激经济上可行的PET塑料升级回收产业的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
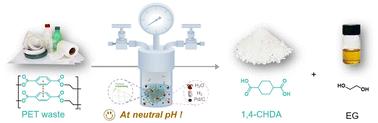
Hydrogenation-induced selective degradation of PET wastes†
Polyethylene terephthalate (PET) is a ubiquitous and versatile thermoplastic polymer that has a wide range of applications in daily life. However, like other synthetic plastics, the large-scale production and accumulation of PET has resulted in a heavy environmental burden. Chemical upcycling is a promising strategy to address the environmental crises posed by PET plastic wastes, yet its practical implementation remains challenging. Herein, we report a novel hydrogenation-induced hydrothermal degradation strategy for upcycling of PET to valuable products. For the first time, a range of highly crystalline post-consumer PET wastes were efficiently converted into high-value 1,4-cyclohexanedicarboxylic acid (1,4-CHDA) and ethylene glycol (EG) in excellent yields under simple neutral water conditions. Detailed mechanistic investigations indicated that hydrogenation of aromatic rings in PET chains occurred preferentially relative to hydrolysis of the ester linkages. The hydrogenation process not only eliminated the intermolecular π–π stacking forces, but also disrupted the intramolecular conjugated π-systems, thus increasing the electrophilic and hydrolytic reactivity of ester linkages in PET. This work opens a new cost-effective route for PET upcycling, which may stimulate the development of an economically viable upcycling industry for PET plastics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


