葡萄糖功能化氧化还原反应型双氢青蒿素前药纳米系统用于疟疾靶向治疗
IF 6.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
虽然疟疾已得到有效控制,但它仍然对全球健康构成威胁。青蒿素是一线抗疟药物。然而,由于溶解度差和生物半衰期短,其治疗效果明显受到限制。为了克服这些限制并增强疟原虫的药物积累,我们开发了葡萄糖功能化氧化还原反应的双氢青蒿素(DHA)前药纳米系统(D@GLU-PMs-SS)。采用dha -二硫代二丙酸-十八乙胺前药和D-α-生育酚聚乙二醇1000琥珀酸熊果苷缀合物制备纳米体系。所得D@GLU-PMs-SS在贮藏条件和生理环境下均表现出良好的稳定性。D@GLU-PMs-SS可以被谷胱甘肽(GSH)激活,导致纳米颗粒解离并随后释放游离DHA。体外实验表明,宿主红细胞对葡萄糖功能化纳米颗粒的摄取通过谷氨酰胺介导的运输显着增强。细胞实验表明D@GLU-PMs-SS有效降低了疟原虫中谷胱甘肽的浓度。此外,D@GLU-PMs-SS在抑制疟原虫生长的同时保持生物安全性方面表现出显著的效果。总的来说,这项研究开发了一种策略来增强纳米颗粒的靶向性,以提高它们对疟疾的治疗效果,值得在临床试验中进一步研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
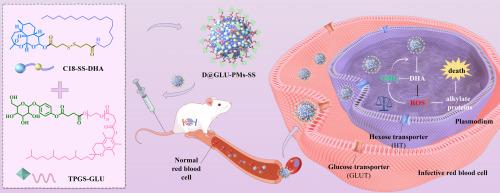
Glucose-functionalized redox-responsive dihydroartemisinin prodrug nanosystem for targeted malaria therapy
Although malaria has been effectively controlled, it still poses a threat to global health. Artemisinins are the first-line antimalarial drugs. However, their therapeutic efficacy is significantly limited by poor solubility and short biological half-life. To overcome these limitations and enhance drug accumulation in Plasmodium, we developed a glucose-functionalized redox-responsive dihydroartemisinin (DHA) prodrug nanosystem (D@GLU-PMs-SS). The nanosystem was prepared by using DHA-dithiodipropionic acid-octadecylamine prodrug and D-α-Tocopherol polyethylene glycol 1000 succinate-arbutin conjugate. The resultant D@GLU-PMs-SS exhibited excellent stability under conditions of storage and physiological environment. D@GLU-PMs-SS could be activated by glutathione (GSH), leading to the dissociation of nanoparticles and subsequent release of free DHA. In vitro experiments revealed that the host erythrocyte uptake of glucose-functionalized nanoparticles was significantly enhanced via GLUT-mediated transport. Cellular experiments illustrated that D@GLU-PMs-SS effectively reduced GSH concentrations in Plasmodium. Furthermore, D@GLU-PMs-SS displayed remarkable efficacy in inhibiting the growth of Plasmodium while maintaining biosafety. Overall, this study developed a strategy to enhance the targeting of nanoparticles to improve their therapeutic efficacy against malaria, warranting further investigation in clinical trials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


