活体荧光原位杂交(Live-FISH)在土壤微生物组上的适用性
IF 10.3
1区 农林科学
Q1 SOIL SCIENCE
引用次数: 0
摘要
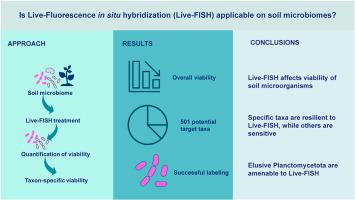
土壤微生物群在生态系统功能中起着关键作用,并已成为重要的商业酶和药物线索的宝库。近年来,宏基因组研究已经揭示了这些微生物组的巨大分类和功能多样性,导致人们认识到大多数微生物仍然无法通过传统的培养方法获得。活体荧光原位杂交(Live-Fluorescence in situ Hybridization, Live-FISH)与荧光激活细胞分选相结合,为克服培养障碍提供了一种潜在的策略,可以通过特定分类提取活细胞进行靶向培养。然而,目前尚不清楚这种方法在多大程度上适用于土壤微生物组。在这里,我们评估了活鱼对温带表土中提取的微生物活力的影响。使用单叠氮丙啶(PMA)活力qPCR,我们观察到一个数量级的减少活细胞的数量作为处理的结果。通过PMA活力测序,我们观察到作为处理的功能,有501个asv保持了活力,可以作为未来培养工作的目标。结果表明,该效应具有分类特异性,Bacillota和plantomycetota的生存力比其他优势门如Acidobacteriota的生存力保持得更大,而Acidobacteriota在整个过程中降低了5个数量级。此外,在随后的显微镜和流式细胞术分析中,plantometes易于标记和区分,证明了该技术在选择性标记和提取这一难以捉摸的门的活物种方面的实用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Applicability of live-fluorescence in situ hybridization (Live-FISH) on soil microbiomes
Soil microbiomes play pivotal roles in ecosystem functioning and have been a trove of commercially important enzymes and drug leads. In recent years, metagenomic scrutiny has exposed the immense taxonomic and functional diversity of these microbiomes, leading to the realization that most microorganisms remain out of reach by conventional cultivation approaches. Live-Fluorescence in situ Hybridization (Live-FISH) coupled with fluorescence activated cell sorting represents a potential strategy to overcome cultivation barriers by enabling the taxa-specific extraction of viable cells for targeted cultivation efforts. However, it remains unknown to what extent this approach is applicable on soil microbiomes. Here, we evaluated the impact of Live-FISH on the viability of microorganisms extracted from temperate topsoil. Using propidium monoazide (PMA) viability qPCR, we observed a one-order of magnitude reduction in the number of viable cells as a result of the treatment. Through PMA viability sequencing, we observed an overall reduction in viable taxa as function of treatment, yet, 501 ASVs retained their viability and could serve as targets for future cultivation efforts. The effects proved to be taxon-specific, and we observed that the viability of Bacillota and Planctomycetota was retained to a larger extent than that of other dominant phyla like Acidobacteriota, which were reduced by five orders of magnitude throughout the steps of the procedure. Furthermore, planctomycetes were amenable to labelling and distinguishable in subsequent microscopy and flow cytometry analyses, demonstrating the utility of this technique to selectively label and extract viable species of this elusive phylum.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Soil Biology & Biochemistry
农林科学-土壤科学
CiteScore
16.90
自引率
9.30%
发文量
312
审稿时长
49 days
期刊介绍:
Soil Biology & Biochemistry publishes original research articles of international significance focusing on biological processes in soil and their applications to soil and environmental quality. Major topics include the ecology and biochemical processes of soil organisms, their effects on the environment, and interactions with plants. The journal also welcomes state-of-the-art reviews and discussions on contemporary research in soil biology and biochemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


