阴离子在层层纳滤膜上的霍夫迈斯特效应:组装动力学和微污染物去除
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
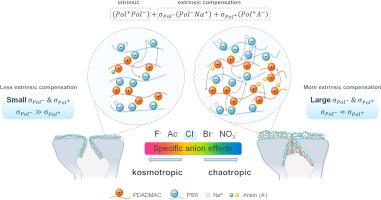
阴离子对纳滤膜组装过程、后续物理化学特性和分离性能的影响尚未完全阐明。本研究选择了聚4-苯乙烯磺酸钠(PSS)和聚二烯基二甲基氯化铵(PDADMAC)的模型聚电解质(PE)对,并将Hofmeister系列中的一价阴离子-宇宙性离子(F−,Ac−),Cl−,乱性离子(Br−,NO3−)与Na+作为背景盐配对。各向异性阴离子制备的膜具有孔径小、分布窄、渗透率较低、接近电中性、甚至略带电正电的分离层;在MgSO4截除中,达到平台所需的涂层双层更少,相应的PE吸附更快,涂层更厚。动态光散射(DLS)技术表明,PE在溶液中的扩散系数比位点扩散系数高几个数量级,表明PE的吸附主要是通过快速位点扩散来增强的。椭偏厚度测量结果表明,PDADMAC在各向异性阴离子上的吸附增量大于PSS,这解释了膜总电荷的差异。超向阴离子膜由于孔径小,在去除药品和个人护理用品(PPCPs)以及单氟烷基和多氟烷基物质(PFASs)方面表现较好,突出了位阻的重要性。背景阴离子在调节PE吸附、调节孔隙结构、电荷和分离性能方面的作用对于未来设计LBL NF膜以满足广泛的分离需求至关重要。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hofmeister effect of anions on the layer-by-layer nanofiltration membranes: Assembly kinetics and micropollutant removal
The impact of anions on the assembly process, subsequent physicochemical characteristics and separation performance of layer-by-layer (LBL) nanofiltration (NF) membranes has not been fully elucidated. In this study, model polyelectrolyte (PE) pairs of poly(sodium 4-styrenesulfonate) (PSS) and poly(diallyldimethylammonium chloride) (PDADMAC) were chosen and monovalent anions in the Hofmeister series—kosmotropes (F−, Ac−), Cl−, chaotropes (Br−, NO3−)—were paired with Na+ as the background salt. Membranes from chaotropic anions had narrow distributions of small pores, relatively low permeance and nearly electroneutral, even slightly electropositive separation layer; fewer coating bilayers were required to reach the plateau in MgSO4 rejection, corresponding to faster PE adsorption and thicker coating. Dynamic light scattering (DLS) technique revealed that the diffusion coefficients of PEs in solution were several magnitudes higher than the site diffusion coefficients, implying that PE adsorption was mainly enhanced by fast site diffusion. Thickness measurement by ellipsometry on silicon wafers demonstrated that the increment of adsorption was greater for PDADMAC than for PSS in chaotropic anions, which explained the difference in the overall membrane charge. Membranes from chaotropic anions performed better in removing pharmaceuticals and personal care products (PPCPs) and per- and poly-fluoroalkyl substances (PFASs) due to small pore size, highlighting the importance of steric exclusion. The role of background anions in modulating PE adsorption, tuning the pore structure, charges and separation performance is of paramount importance for future design of LBL NF membranes for a broad spectrum of separation needs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


