乌曲西兰对HELIOS-B型转甲状腺素淀粉样变合并心肌病患者心脏生物标志物的影响
IF 22.3
1区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
背景:在转甲状腺素淀粉样变性心肌病(atr - cm)的疾病改善疗法发展之前,b型利钠肽n端原激素(NT-proBNP)和肌钙蛋白I/T被认为是死亡率的独立预后生物标志物。本研究评估了这些生物标志物在当代患者群体中的预后价值,以及vutrisiran对生物标志物水平的影响。vutrisiran是一种RNA干扰疗法,可快速降低循环甲状腺素转运。目的:本研究旨在评估HELIOS-B患者6个月时基线NT-proBNP和肌钙蛋白I水平变化与心血管事件风险和全因死亡率之间的关系,并探讨vutrisiran如何随时间影响生物标志物。方法在HELIOS-B双盲、安慰剂对照研究中,655例atr - cm患者以1:1的比例随机分组,接受乌崔西兰或安慰剂治疗,疗程长达36个月。主要终点是全因死亡率和心血管事件复发的复合结局。42个月的全因死亡率是次要终点。NT-proBNP和肌钙蛋白I被评估为预先指定的探索性终点。结果基线NT-proBNP和肌钙蛋白I水平与复合结局和全因死亡率风险独立相关(P <;生物标志物和终点均为0.0001)。在第6个月,NT-proBNP从基线增加与复合结局和全因死亡的高风险相关,肌钙蛋白I降低与复合结局的风险降低相关。在第30个月,安慰剂组NT-proBNP和肌钙蛋白I从基线变化的中位数分别为753 pg/mL (Q1-Q3:−8至2,573 pg/mL)和9.7 pg/mL (Q1-Q3:−6.3至41.2 pg/mL), vutrisiran组为118 pg/mL (Q1-Q3:−419至911 pg/mL)和−5.8 pg/mL (Q1-Q3:−25.0至10.0 pg/mL)。NT-proBNP组的几何平均折数变化比(vutrisiran/安慰剂组)为0.68 (95% CI: 0.61-0.76),肌钙蛋白I组为0.68 (95% CI: 0.62-0.75)。两者均为0.0001)。结论:生物标志物与不良结局之间的关联模式支持早期治疗的重要性以及对atr - cm患者风险降低的潜力。Vutrisiran维持稳定或降低了这两种生物标志物的水平,这与治疗在降低心血管事件风险和全因死亡率方面的益处一致。HELIOS-B: vtrisiran在转甲状腺素淀粉样变合并心肌病患者中的应用研究NCT04153149)本文章由计算机程序翻译,如有差异,请以英文原文为准。
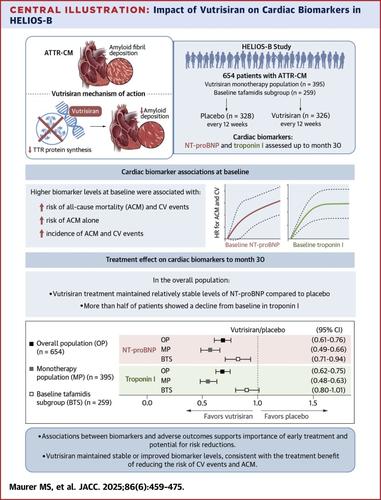
Impact of Vutrisiran on Cardiac Biomarkers in Patients With Transthyretin Amyloidosis With Cardiomyopathy From HELIOS-B
Background
Before the development of disease-modifying therapies for transthyretin amyloidosis cardiomyopathy (ATTR-CM), N-terminal prohormone of B-type natriuretic peptide (NT-proBNP) and troponin I/T were recognized as independent prognostic biomarkers of mortality. This study evaluated the prognostic value of these biomarkers in a contemporary patient population and the impact of vutrisiran, an RNA interference therapeutic that rapidly knocks down circulating transthyretin, on biomarker levels.
Objectives
This study sought to evaluate the association between risk of cardiovascular events and all-cause mortality with baseline NT-proBNP and troponin I levels and changes from baseline at month 6 in patients from HELIOS-B and explore how vutrisiran impacts biomarkers over time.
Methods
In HELIOS-B, a double-blind, placebo-controlled study, 655 patients with ATTR-CM were randomized 1:1 to receive vutrisiran or placebo for up to 36 months. The primary endpoint was a composite outcome of all-cause mortality and recurrent cardiovascular events. All-cause mortality through 42 months was a secondary endpoint. NT-proBNP and troponin I were assessed as prespecified exploratory endpoints.
Results
Baseline NT-proBNP and troponin I levels were independently associated with risks of the composite outcome and all-cause mortality (P < 0.0001 for both biomarkers and endpoints). At month 6, increases in NT-proBNP from baseline were associated with higher risk of the composite outcome and all-cause mortality, and decreases in troponin I were associated with a lower risk of the composite outcome. At month 30, the median changes from baseline of NT-proBNP and troponin I were 753 pg/mL (Q1-Q3: −8 to 2,573 pg/mL) and 9.7 pg/mL (Q1-Q3: −6.3 to 41.2 pg/mL) in the placebo arm and 118 pg/mL (Q1-Q3: −419 to 911 pg/mL) and −5.8 pg/mL (Q1-Q3: −25.0 to 10.0 pg/mL) in the vutrisiran arm. The geometric mean fold-change ratios (vutrisiran/placebo) were 0.68 (95% CI: 0.61-0.76) for NT-proBNP and 0.68 (95% CI: 0.62-0.75) for troponin I (P < 0.0001 for both).
Conclusions
Patterns of associations between biomarkers and adverse outcomes support the importance of early treatment initiation and the potential for risk reduction in patients with ATTR-CM. Vutrisiran maintained stable or reduced levels of both biomarkers consistent with the benefit of treatment in reducing the risk of cardiovascular events and all-cause mortality. (HELIOS-B: A Study to Evaluate Vutrisiran in Patients With Transthyretin Amyloidosis With Cardiomyopathy; NCT04153149)
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
42.70
自引率
3.30%
发文量
5097
审稿时长
2-4 weeks
期刊介绍:
The Journal of the American College of Cardiology (JACC) publishes peer-reviewed articles highlighting all aspects of cardiovascular disease, including original clinical studies, experimental investigations with clear clinical relevance, state-of-the-art papers and viewpoints.
Content Profile:
-Original Investigations
-JACC State-of-the-Art Reviews
-JACC Review Topics of the Week
-Guidelines & Clinical Documents
-JACC Guideline Comparisons
-JACC Scientific Expert Panels
-Cardiovascular Medicine & Society
-Editorial Comments (accompanying every Original Investigation)
-Research Letters
-Fellows-in-Training/Early Career Professional Pages
-Editor’s Pages from the Editor-in-Chief or other invited thought leaders

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


