UiO-66对提高MgH2储氢性能的催化作用
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
本研究通过加入锆基金属有机骨架(MOF) UiO-66,证明了氢化镁(MgH2)的储氢性能得到了改善。UiO-66的加入显著提高了吸附动力学,并将分解温度降低到400℃以下。UiO-66通过溶剂热法合成,在300°C下煅烧后稳定,具有优异的热稳定性和化学稳定性,是一种很有前途的储氢添加剂。MgH2/ uuo -66复合材料的初始脱氢温度为262℃,比MgH2粉磨后的脱氢温度低80℃。表观活化能降低到85.5±5.5 kJ/mol,约为原始MgH2的45%,表明反应途径明显增强。在250°C时,该复合材料在3600秒内达到约6.8 wt%的氢容量,并在连续10次循环中保持稳定的性能。通过扫描电子显微镜(SEM)的粒度分析显示,与单独研磨的MgH2相比,复合材料中的分散更细,团聚减少。MgH2/UiO-66系统有效地充当“氢泵”,促进更快的加氢/脱氢动力学,提高循环稳定性。因此,这项研究为扩大研究和加速氢能的发展提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
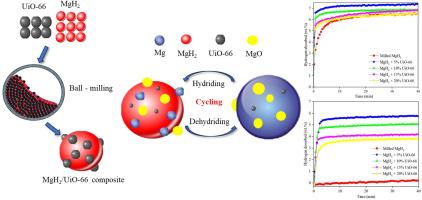
The catalytic effects of UiO-66 on enhancing hydrogen storage performance of MgH2
This study demonstrates the improved hydrogen storage performance of magnesium hydride (MgH2) through the incorporation of a zirconium-based metal-organic framework (MOF), UiO-66. The addition of UiO-66 significantly enhances the sorption kinetics and reduces the decomposition temperature to below 400 °C. Synthesized via a solvothermal route and stabilized by post-calcination at 300 °C, UiO-66 exhibits excellent thermal and chemical stability, making it a promising additive for hydrogen storage systems. The MgH2/UiO-66 composite shows an initial dehydrogenation temperature of 262 °C, which is 80 °C lower than that of milled MgH2. The apparent activation energy is reduced to 85.5 ± 5.5 kJ/mol, approximately 45 % of the pristine MgH2, indicating a significantly enhanced reaction pathway. At 250 °C, the composite achieves a hydrogen capacity of approximately 6.8 wt% within 3600 s and maintains stable performance over ten consecutive cycles. Particle size analysis via scanning electron microscopy (SEM) reveals finer dispersion and reduced agglomeration in the composite compared to milled MgH2 alone. The MgH2/UiO-66 system effectively functions as a “hydrogen pump,” facilitating faster hydrogenation/dehydrogenation kinetics and improved cycling stability. Hence, this study offers fresh insights to expand research and accelerate the advancement of hydrogen energy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


