用于测量细胞和组织力学各向异性的环形压痕。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
基于压痕的机械测试有利于测量组织和细胞的刚度,因为它们简单且能够无损地探测样品。最常见的是使用球形或锥体探针,并应用赫兹分析来计算模量。该技术假设材料各向同性,忽略了纤维组织和极化细胞的方向依赖特性。在这项研究中,我们旨在开发一种广义的压痕方法来估计生物材料从宏观组织到单个细胞的各向异性弹性模量。环形压头探头的半径范围从毫米到微米,纵横比高达10:1。各向异性肌肉组织、细胞单层和单细胞在垂直和平行于其首选纤维方向的方向上缩进。为了确定材料的本征各向异性模量(E1和E2),建立了线性不可压缩横向各向同性材料模型,并采用有限元方法模拟了E1:E2参数组下归一化加载曲线对。然后使用模拟数据训练深度学习模型,并用于计算模量。我们发现,各向异性的程度(E1:E2)与已发表的结果相当,肌肉(~ 1:37 .7)、排列的单层细胞(~ 1:36 .6)和极化的单细胞(~ 1:17 .7)。该方法推广了各向异性生物材料和细胞的各向同性赫兹压痕方法。环形探头的制造成本不到30美元,并且与商业压头兼容,使研究人员广泛使用该方法。意义声明:本研究中开发的环形压痕方法和基于有限元的深度学习模型提供了一种易于获得、易于获取和低成本的方法,可以使用传统的实验室设备测量从宏观组织到微观细胞的广泛生物材料的各向异性刚度,据我们所知,如果不使用成像和逆有限元模型或高度专业化的定制设备,这是不可能的。通过提供一种工具,本研究有可能促进对极化细胞、多细胞聚集体和微组织等各向异性生物系统的力学特性的理解,从而加快组织工程、生物力学和力学生物学的研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
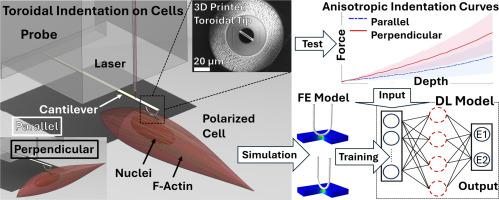
Toroidal indentation for measuring cell and tissue mechanical anisotropy
Indentation-based mechanical tests are advantageous for measuring tissue and cell stiffness due to their simplicity and ability to probe samples non-destructively. Most commonly, spherical or pyramidal probes are used, and Hertzian analysis is applied to calculate the modulus. This technique assumes material isotropy, which ignores the direction-dependent properties of fibrous tissues and polarized cells. In this study, we aimed to develop a generalized indentation method to estimate the anisotropic elastic moduli of biomaterials across scales from macroscopic tissues to single cells. Torus-shaped indenter probes were created with radii ranging from millimeters to microns and aspect ratios up to 10:1. Anisotropic muscle tissue, cell monolayers, and single cells were indented in directions perpendicular and parallel to their preferred fiber orientations. To determine intrinsic anisotropic moduli (E1 and E2), a linear incompressible transversely isotropic material model was developed, and finite element modeling was used to simulate normalized loading curve pairs for a wide range of E1:E2 parameter sets. A deep learning model was then trained with the simulated data and used to calculate the moduli. We found that the degree of anisotropy (E1:E2) was comparable to published results for muscle (∼1:3.7), aligned cell monolayers (∼1:3.6), and polarized single cells (∼1:1.7). This method generalizes the isotropic Hertzian indentation approach for anisotropic biomaterials and cells across length scales. The toroidal probes can be fabricated for less than $30 and are compatible with commercial indenters, making the method widely accessible to researchers.
Statement of significance
The toroidal indentation method and the finite element based deep learning model developed in this study provides a readily available, accessible, and low-cost method to measure anisotropic stiffness of broad biological materials from macroscopic tissues to microscopic cells using conventional laboratory devices, which was, to the best of our knowledge, impossible without using imaging and inverse finite element model or highly specialized customized equipment. By providing a tool, this study has the potential to facilitate the understanding of mechanical properties of anisotropic biological systems such as polarized cells, multicellular aggregates, and micro tissues, hence expedite the studies in tissue engineering, biomechanics, and mechanobiology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


