卢旺达是如何在马尔堡疫情爆发10天后,用一种研究性疫苗开展研究应对的。
IF 6.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
摘要
2024年9月暴发的马尔堡病毒病是卢旺达首次发现这种病毒。卫生当局采取了迅速而有效的应对措施,包括在宣布疫情暴发后仅10天就迅速开展了一种研究性马尔堡疫苗的开放标签第二期临床试验。我们分享这一快速实施的临床试验的经验教训,以帮助鼓励其他国家做好疫情研究准备。本文章由计算机程序翻译,如有差异,请以英文原文为准。
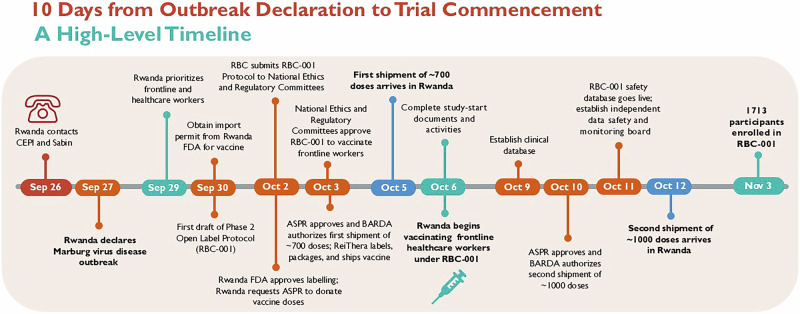
How Rwanda mounted a research response with an investigational vaccine just ten days into a Marburg outbreak.
The September 2024 outbreak of Marburg virus disease (MVD) represented the first time this virus was identified in Rwanda. Health authorities mounted a rapid and effective response, including the rapid execution of an open-label Phase 2 clinical trial of an investigational Marburg vaccine, beginning just 10 days after the outbreak was declared. We share lessons from this rapidly executed clinical trial to help encourage outbreak research preparedness in other countries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

NPJ Vaccines
Immunology and Microbiology-Immunology
CiteScore
11.90
自引率
4.30%
发文量
146
审稿时长
11 weeks
期刊介绍:
Online-only and open access, npj Vaccines is dedicated to highlighting the most important scientific advances in vaccine research and development.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


