亚硝化应激影响酿酒酵母线粒体呼吸链复合体II和复合体IV组装:复合体II的s -亚硝基化。
IF 2.2
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. General subjects
Pub Date : 2025-07-30
DOI:10.1016/j.bbagen.2025.130845
引用次数: 0
摘要
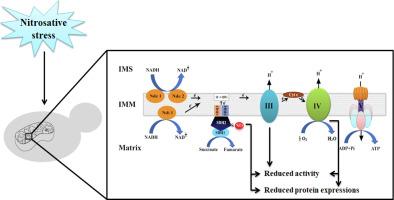
一氧化氮(NO)和活性氮(RNS)水平升高引起细胞亚硝化应激,抑制线粒体呼吸。报告显示,RNS在分离的线粒体中迅速灭活复合体I,随后抑制复合体II、III和IV。然而,NO和RNS抑制这些复合物的机制尚不清楚。在本研究中,我们利用兼性厌氧酵母研究亚硝化应激下的线粒体呼吸功能障碍,因为5个线粒体氧化磷酸化复合物中有4个,即复合物II、III、IV和V在结构上从酵母到人类是保守的。通过微生物生长试验,研究人员发现,用梯度浓度的硝普钠(SNP)和s -亚硝基谷胱甘肽(GSNO)处理酿酒酵母野生型W3O3细胞可诱导亚硝化应激,细胞生长在富含甘油-乙醇的呼吸系统培养基中受到严重损害。整个细胞和线粒体耗氧量在亚硝化胁迫下也显著降低。令人惊讶的是,酵母线粒体呼吸链复合体II琥珀酸脱氢酶(SDH)在亚硝化胁迫下被发现s -亚硝基化,因此失活。酿酒酵母s -亚硝基谷胱甘肽还原酶突变细胞产生的内源性RNS也显示SDH的s -亚硝基化增加。在亚硝化胁迫下,酿酒酵母复合物III和IV活性不可逆地受到抑制。有趣的是,在SNP处理后,线粒体中的蛋白酪氨酸硝化也以剂量依赖的方式增强。在亚硝酸盐胁迫下,Sdh2(琥珀酸脱氢酶亚基-2)和Cox2(线粒体复合体IV亚基)在转录和翻译水平上的表达均有所降低。Blue native - page随后进行Western blotting分析,进一步揭示了在亚硝化胁迫下葡萄球菌的天然复合物II和含有超复合物组装体的复合物III和IV显著减少。因此,目前的体内研究首次提供了亚硝化应激下线粒体复合物修饰的新信息,这反过来调节了酿酒酵母线粒体呼吸链复合物的组装。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nitrosative stress affects mitochondrial respiratory chain complex II and complex IV assemblies in Saccharomyces cerevisiae: S-nitrosylation of complex II
The elevated level of nitric oxide (NO) and reactive nitrogen species (RNS) induce nitrosative stress in cells and inhibit mitochondrial respiration. Reports showed that RNS rapidly inactivate complex I, followed by inhibition of complex II, III and IV in isolated mitochondria. However, the mechanism(s) by which NO and RNS inhibit these complexes still unclear. In this study facultative anaerobic yeast Saccharomyces cerevisiae has been used for investigating mitochondrial respiratory dysfunction under nitrosative stress, as four out of five mitochondrial oxidative phosphorylation complexes i.e. complexes II, III, IV and V are structurally conserved from yeast to human. Using microbiological growth assays, we showed that S. cerevisiae wild type W3O3 cells treated with graded concentration of sodium nitroprusside (SNP) and S-Nitrosoglutathione (GSNO) induce nitrosative stress, and cell growth was severely compromised under the respiratory proficient rich glycerol-ethanol media. Both the whole cell and the mitochondrial oxygen consumption rates were also significantly compromised under nitrosative stress. Surprisingly, mitochondrial respiratory chain complex II succinate dehydrogenase (SDH) of S. cerevisiae was found S-nitrosylated and therefore inactivated under nitrosative stress. Endogenous RNS produced by S-nitrosoglutathione reductase mutant cells of S. cerevisiae also showed increased S-nitrosylation of SDH. Complex III and IV activities were irreversibly inhibited in S. cerevisiae under nitrosative stress. Interestingly, protein tyrosine nitration was also enhanced in mitochondria in a dose dependent manner upon SNP treatment. Reduced expressions of both Sdh2 (succinate dehydrogenase subunit-2) and Cox2 (mitochondrial complex IV subunit) were observed at the transcription and translation level in S. cerevisiae under nitrosative stress. Blue Native-PAGE followed by Western blotting analysis, further revealed significantly reduced native complex II and the complex III and IV containing super-complexes assemblies in consequences of nitrosative stress in S. cerevisiae. Henceforth, the present in vivo study provides for the first-time novel information on the modification of mitochondrial complexes under nitrosative stress which in turn regulates the mitochondrial respiratory chain complexes assembly in S. cerevisiae.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochimica et biophysica acta. General subjects
生物-生化与分子生物学
CiteScore
6.40
自引率
0.00%
发文量
139
审稿时长
30 days
期刊介绍:
BBA General Subjects accepts for submission either original, hypothesis-driven studies or reviews covering subjects in biochemistry and biophysics that are considered to have general interest for a wide audience. Manuscripts with interdisciplinary approaches are especially encouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


