多组学分析为肠道真菌适应膳食碳水化合物的机制提供了见解
IF 5.8
Q1 MICROBIOLOGY
引用次数: 0
摘要
碳水化合物是人类和动物饮食中必不可少的能量来源,但肠道真菌利用碳水化合物的机制尚不清楚。为了解决这一差距,我们采用白色念珠菌——人类和猪普遍存在的肠道真菌物种——作为模型来研究真菌对碳水化合物的利用策略。利用整合转录组学和代谢组学分析的多组学方法,我们研究了真菌在体外发酵过程中的生长动态、碳水化合物降解模式和酶活性。我们的研究结果表明,白色念珠菌优先利用可溶性多糖,如菊粉和甘露寡糖(MOS),而对淀粉的降解效率较低。综合转录组学和代谢组学分析发现了与碳水化合物代谢相关的不同代谢物和差异表达基因,并观察到碳水化合物活性酶(CAZymes)与特定代谢中间体之间存在很强的相关性。值得注意的是,CAZyme表达式substrate-dependent:菊粉特别诱导糖苷水解酶家族15 (GH15 EC 3.2.1.3),目标α1,2-glycosidic联系,而MOS调节更广泛的enzymes-including GH13_40 (EC 3.2.1.10), GH15, GH16_2 (EC 3.2.1 - / 2.4.1 -)和GH17 (EC 3.2.1.58/2.4.1 -),作用于β1,4 -,α1,6 -,α1,2 -,和α1,3-glycosidic债券、协调高效的复合碳水化合物胞外水解成可吸收的单糖。这项研究强调了肠道真菌在膳食碳水化合物利用中的关键作用,并为CAZymes介导真菌碳水化合物代谢的机制提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
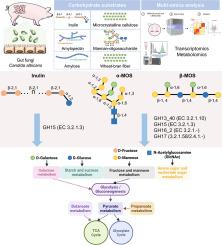
Multi-omics analysis provides insights into mechanisms of intestinal fungi adaptation to dietary carbohydrates
Carbohydrates are essential energy sources in the diets of humans and animals, yet the mechanisms underlying their utilization by gut fungi remain poorly understood. To address this gap, we employed Candida albicans—a prevalent gut fungal species in humans and pigs—as a model to investigate fungal carbohydrate utilization strategies. Using a multi-omics approach integrating transcriptomic and metabolomic analyses, we examined fungal growth dynamics, carbohydrate degradation patterns, and enzyme activity during in vitro fermentation. Our results revealed that C. albicans preferentially utilizes soluble polysaccharides, such as inulin and mannan-oligosaccharides (MOS), while exhibiting lower efficiency in degrading starch. Integrated transcriptomic and metabolomic analyses identified distinct metabolites and differentially expressed genes associated with carbohydrate metabolism, with strong correlations observed between carbohydrate-active enzymes (CAZymes) and specific metabolic intermediates. Notably, CAZyme expression was substrate-dependent: inulin specifically induced glycoside hydrolase family 15 (GH15, EC 3.2.1.3), which targets α-1,2-glycosidic linkages, whereas MOS upregulated a broader set of enzymes—including GH13_40 (EC 3.2.1.10), GH15, GH16_2 (EC 3.2.1-/2.4.1-) and GH17 (EC 3.2.1.58/2.4.1-) — that act on β-1,4-, α-1,6-, α-1,2-, and α-1,3-glycosidic bonds, mediating efficient extracellular hydrolysis of complex carbohydrates into absorbable monosaccharides. This study highlights the critical role of gut fungi in dietary carbohydrate utilization and provides novel insights into the mechanisms by which CAZymes mediate fungal carbohydrate metabolism.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Current Research in Microbial Sciences
Immunology and Microbiology-Immunology and Microbiology (miscellaneous)
CiteScore
7.90
自引率
0.00%
发文量
81
审稿时长
66 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


