邻二碘苯/萘为苄基体系由环戊二烯酮合成多取代萘
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
提出了一种环戊二酮衍生物与苯之间Diels-Alder环加成合成多取代萘的有效方法。利用邻二碘苯作为稳定的苯前体与氢化钠(NaH)结合,可以方便地原位生成活性苯中间体。该方法具有操作简单、原料易得、成本低廉、对多种官能团的耐受性等特点,为合成多环芳香族结构提供了一个环境友好的平台,在材料科学和药物化学领域具有潜在的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
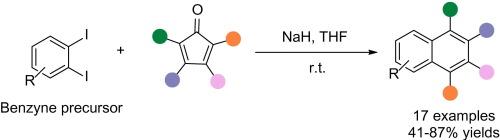
Synthesis of polysubstituted naphthalenes from cyclopentadienones using o-diiodobenzene/NaH as a benzyne system
We present an efficient strategy for constructing polysubstituted naphthalenes through Diels-Alder cycloaddition between cyclopentadienone derivatives and benzynes. Utilizing o-diiodobenzene as a stable benzyne precursor in combination with sodium hydride (NaH) enables facile in situ generation of reactive benzyne intermediate. The method features operational simplicity, use of readily accessible and cost-effective raw materials and tolerance towards diverse functional groups, thereby establishing an environmentally benign platform for synthesizing polycyclic aromatic architectures with potential applications in materials science and pharmaceutical chemistry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


