羰基二咪唑催化溴化苄基的亲核三氟甲氧基化
IF 2.7
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
提出了一种操作简单的亲核三氟甲氧基化方案,通过廉价、市售和稳定的试剂,羰基二咪唑和AgF。机理研究表明,该方法不通过传统的三氟甲氧基阴离子途径,产生有毒的二氟光气作为中间体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
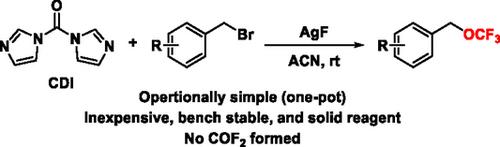
Nucleophilic Trifluoromethoxylation of Benzyl Bromides via Carbonyl Diimidazole
An operationally simple nucleophilic trifluoromethoxylation protocol via an inexpensive, commercially available and bench stable reagent, carbonyl diimidazole and AgF is presented. Mechanistic studies are performed to reveal that this method does not proceed through the conventional trifluoromethoxide anion pathway that generates toxic difluorophosgene as an intermediate.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
5.40
自引率
3.60%
发文量
752
审稿时长
1 months
期刊介绍:
The European Journal of Organic Chemistry (2019 ISI Impact Factor 2.889) publishes Full Papers, Communications, and Minireviews from the entire spectrum of synthetic organic, bioorganic and physical-organic chemistry. It is published on behalf of Chemistry Europe, an association of 16 European chemical societies.
The following journals have been merged to form two leading journals, the European Journal of Organic Chemistry and the European Journal of Inorganic Chemistry:
Liebigs Annalen
Bulletin des Sociétés Chimiques Belges
Bulletin de la Société Chimique de France
Gazzetta Chimica Italiana
Recueil des Travaux Chimiques des Pays-Bas
Anales de Química
Chimika Chronika
Revista Portuguesa de Química
ACH—Models in Chemistry
Polish Journal of Chemistry.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


