新生猪心脏中sus-PSMB7_0001作为潜在促血管生成环状RNA的鉴定
IF 4.7
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
摘要
虽然已经在鱼类和啮齿动物心脏中发现了调节血管生成的环状rna (circRNAs),但它们在猪心脏中的表达谱在很大程度上仍然未知。这项研究的目的是确定在出生后的猪心脏中调节血管生成的环状rna。猪出生后第1天(P1)、第3天(P3)、第7天(P7)和第28天(P28)采集的心脏组织的总RNA测序数据此前有报道。本研究分析了数据库中与血管生成相关的保守环状rna。在人脐静脉内皮细胞(HUVECs)和hipsc衍生的内皮细胞(hiPSC-ECs)中进行了功能研究。通过sirna介导的circrna和mirna的敲低来验证它们在调节血管生成中的功能。荧光原位杂交检测circRNA的定位。与P1和P3相比,Sus-PSMB7_0001在猪心脏P7和P28的表达增加。hsa-PSMB7_0025 (sus-PSMB7_0001的人类同源物)的敲低会损害HUVECs和hipsc - ec中DNA的合成、有丝分裂、迁移和管的形成。hsa-PSMB7_0025负向调控hsa-miR-490-3p。激活hsa-miR-490-3p抑制hiPSC-EC的增殖,而抑制hsa-miR-490-3p则促进增殖。抑制miR-490-3p上调hsa-PSMB7_0025。miR-490-3p调控5种下游效应物(TP53BP1、TMOD3、CDYL2、FOXO1和TGFBR1)参与细胞周期和血管调节。这些发现表明circRNA sus-PSMB7_0001是新生猪心脏中潜在的促血管生成分子。本文章由计算机程序翻译,如有差异,请以英文原文为准。
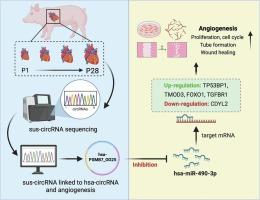
Identification of sus-PSMB7_0001 as a potential pro-angiogenic circular RNA in neonatal pig hearts
While circular RNAs (circRNAs) regulating angiogenesis have been identified in fish and rodent hearts, their expression profiles in pig hearts remain largely unknown. This study aims to identify circRNAs that regulate angiogenesis in postnatal pig hearts. Total RNA sequencing data on pig heart tissues collected on postnatal days 1 (P1), 3 (P3), 7 (P7) and 28 (P28) were previously reported. This study analyzed conserved circRNAs associated with angiogenesis in the database. Functional studies were conducted in human umbilical vein endothelial cells (HUVECs) and hiPSC-derived endothelial cells (hiPSC-ECs). siRNA-mediated knockdown of circRNAs and miRNAs was performed to validate their functions in regulating angiogenesis. Fluorescence in situ hybridization was used to examine circRNA localization. Sus-PSMB7_0001 expression increased in pig hearts at P7 and P28 compared to P1 and P3. Knockdown of hsa-PSMB7_0025 (the human orthologue of sus-PSMB7_0001) impaired DNA synthesis, mitosis, migration, and tube formation in HUVECs and hiPSC-ECs. hsa-PSMB7_0025 negatively regulated hsa-miR-490-3p. Activation of hsa-miR-490-3p inhibited hiPSC-EC proliferation, while its inhibition promoted proliferation. Inhibition of miR-490-3p upregulated hsa-PSMB7_0025. miR-490-3p regulates five downstream effectors (TP53BP1, TMOD3, CDYL2, FOXO1, and TGFBR1) involved in cell cycle and vascular function. These findings suggest circRNA sus-PSMB7_0001 is a potential pro-angiogenic molecule in neonatal pig hearts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


