紫外光促进十钨酸钠催化偶氮苯转移加氢制腙
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
报道了一种以十钨酸钠(NaDT)为催化剂,将偶氮苯高效、选择性地转移加氢制备腙苯的新方法。室温下,在异丙醇(2-PrOH)存在和390 nm紫外光照射下,反应可以顺利进行。对称偶氮苯和不对称偶氮苯在25 ~ 960 min内选择性还原可制得相应的40种加氢偶氮苯,收率可达98%。该反应在不改变反应条件和反应活性的情况下,易于应用于克级实验。而且,与最近报道的同类反应相比,该反应具有最高的催化效果。进一步的机理研究表明,2-PrOH为反应提供氢源,并通过自由基途径实现转化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
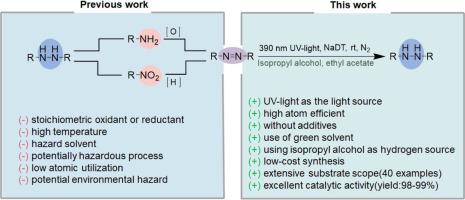
UV-light-promoted transfer hydrogenation of azobenzenes to hydrazobenzenes catalyzed by sodium decatungstate
A novel method of highly efficient and selective transfer hydrogenation of azobenzenes to hydrazobenzenes catalyzed by sodium decatungstate (NaDT) has been reported for the first time. The reaction can be smoothly proceeded in the presence of isopropyl alcohol (2-PrOH) and upon 390 nm UV-light irradiation at room temperature. The corresponding 40 kinds of hydrogenated azobenzene can be obtained by selective reduction of either symmetrical or asymmetrical azobenzene in 25–960 min, and the yield can reach >98 %. Additionally, this reaction can be easily applied in gram-scale experiment without any changes in reaction conditions and reaction activity. Moreover, this reaction has the most catalytic effect compared with similar reactions reported recently. Furthermore, the mechanism study shows that 2-PrOH provides hydrogen source for the reaction and realizes the transformation through free radical pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


