揭示过渡金属掺杂煤焦体系催化析氢反应的多功能性:第一性原理方法
IF 2.4
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
过渡金属掺杂工程煤焦(TMdopChar)系统利用其定制的几何、电子和量子化学性质,已成为析氢反应(HER)的特殊电催化剂。短的TM-H键长度(1.4-1.8 Å)促进了高效的氢吸附和HH偶联,而自旋态的适应性提高了ScdopChar、TidopChar和NidopChar等体系的催化灵活性。量子化学分析表明,这些体系中有利的低能隙(Eg)和HOMO-LUMO排列驱动了优异的催化性能。吸附和QTAIM研究表明,H@ZndopChar表现出最佳的吸附-解吸动力学,Tafel机制的吉布斯自由能(ΔGH)最低为0.036 eV,在HER效率方面优于现有的电催化剂。TM@Char系统的多功能性因其平衡强共价和非共价相互作用的能力而突出,使其成为可持续制氢技术的有希望的候选者。本文章由计算机程序翻译,如有差异,请以英文原文为准。
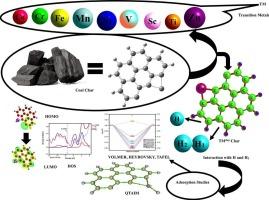
Unveiling the catalytic versatility of transition metal-doped coal char systems for hydrogen evolution reaction: a first-principles approach
Transition metal-doped engineered coal char (TMdopChar) systems have emerged as exceptional electrocatalysts for hydrogen evolution reaction (HER), leveraging their tailored geometric, electronic, and quantum chemical properties. Short TM-H bond lengths (1.4–1.8 Å) promote efficient hydrogen adsorption and H![]() H coupling, while spin state adaptability enhances catalytic flexibility in systems like ScdopChar, TidopChar, and NidopChar. Quantum chemical analyses revealed that favorable low energy gaps (Eg) and HOMO-LUMO alignments in these systems drive superior catalytic performance. Adsorption and QTAIM studies demonstrated that H@ZndopChar exhibits optimal adsorption-desorption dynamics, with the lowest Gibbs free energy (ΔGH) of 0.036 eV for the Tafel mechanism, outperforming established electrocatalysts in HER efficiency. The versatility of TM@Char systems is highlighted by their ability to balance strong covalent and non-covalent interactions, making them promising candidates for sustainable hydrogen production technologies.
H coupling, while spin state adaptability enhances catalytic flexibility in systems like ScdopChar, TidopChar, and NidopChar. Quantum chemical analyses revealed that favorable low energy gaps (Eg) and HOMO-LUMO alignments in these systems drive superior catalytic performance. Adsorption and QTAIM studies demonstrated that H@ZndopChar exhibits optimal adsorption-desorption dynamics, with the lowest Gibbs free energy (ΔGH) of 0.036 eV for the Tafel mechanism, outperforming established electrocatalysts in HER efficiency. The versatility of TM@Char systems is highlighted by their ability to balance strong covalent and non-covalent interactions, making them promising candidates for sustainable hydrogen production technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


