可扩展的超长效替诺福韦磷酸盐前药维持HBV抑制
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
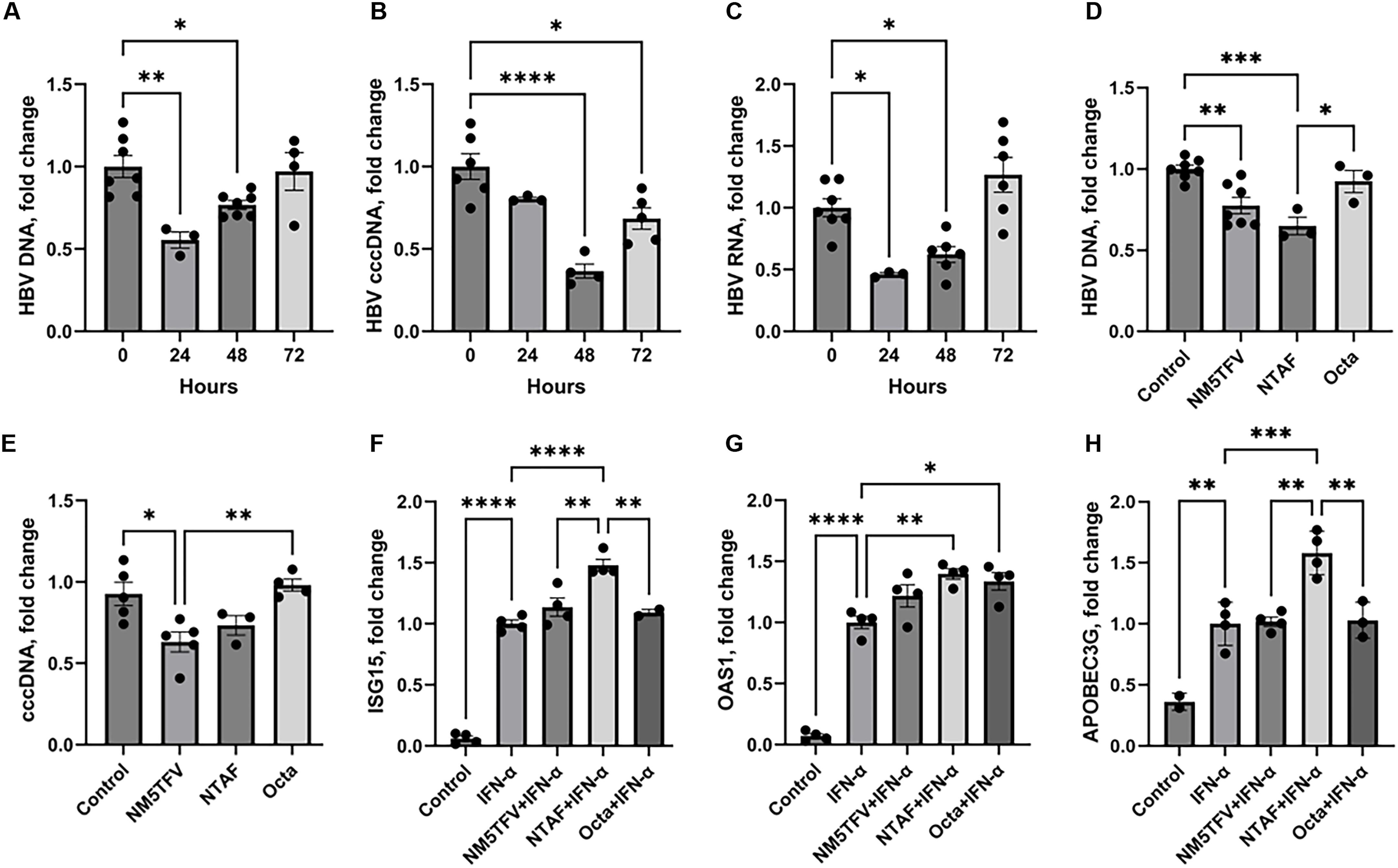
长效(LA)缓释制剂是革命性的治疗和预防艾滋病毒感染。然而,没有一种现有的LA疗法对乙型肝炎病毒(HBV)有效,HBV是一种常见的HIV合并感染。管理合并感染需要对两种病毒都有效的治疗。值得注意的候选药物是替诺福韦(TFV)前药。我们以前通过改良的亲脂性ProTide策略开发了LA TFV。考虑到氨基酸手性中心在氨基酸中的应用带来的工艺化学挑战,我们制备了一种简化的无氨基酸的亲脂性晶体膦酸盐TFV前药(M5TFV)。肌肉注射M5TFV纳米混悬液(NM5TFV)在Sprague-Dawley大鼠和HBV转基因小鼠中具有良好的耐受性。值得注意的是,在转基因小鼠中,200和400毫克/千克TFV当量的单次剂量在2个月后使HBV DNA减少了2.5 log 10。在肝细胞样细胞中可见共价闭合环状DNA的减少。这些有希望的发现支持了NM5TFV作为超la制剂的进一步开发。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A scalable ultra-long-acting tenofovir phosphonate prodrug sustains HBV suppression
Long-acting (LA) extended-release formulations are revolutionizing treatment and prevention of HIV infection. However, none of the existing LA therapies are active against hepatitis B virus (HBV), a common coinfection with HIV. Managing coinfection requires therapy to be effective against both viruses. Notable candidates are tenofovir (TFV) prodrugs. We have previously developed an LA TFV through a modified lipophilic ProTide strategy. Given the process chemistry challenges presented by amino acid chiral centers in ProTides, a simplified lipophilic amino acid–free crystalline phosphonate prodrug of TFV (M5TFV) has been created. Intramuscular injections of M5TFV nanosuspension (NM5TFV) were well tolerated in Sprague-Dawley rats and HBV transgenic mice. Notably, single doses at 200 and 400 milligrams per kilogram TFV equivalents produced >2.5 log10 reduction in HBV DNA beyond 2 months in transgenic mice. Reductions of covalently closed circular DNA were seen in hepatocyte-like cells. These promising findings support further development of NM5TFV as an ultra-LA formulation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


