胶质细胞衍生的非典型脂肪酸结合蛋白调节脑脂质储存和清除
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
胶质细胞衍生的分泌因子对大脑发育、生理和体内平衡至关重要,其功能障碍与各种神经系统疾病有关。通过遗传和生化方法,我们鉴定了一种在果蝇中枢神经胶质中高度表达的非典型α-螺旋脂肪酸结合蛋白(FABP)——气味结合蛋白44a (Obp44a)。Obp44a结合长链脂肪酸,穿梭于胶质细胞和神经元之间,作为分泌脂质伴侣和清道夫,支持脂质储存、外排和氧化还原稳态。值得注意的是,Obp44a被募集到凋亡细胞和受损轴突上,特别是当胶质吞噬受损时,这表明它在发育和病理状态下参与脂质废物管理和细胞碎片清除。我们的研究结果强调了FABPs在调节脑脂质动力学和神经元对应激和损伤的反应中的重要性。通过观察FABP在体内的功能,本研究提供了关于脂质调节缺陷如何促进神经元应激和疾病进展的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
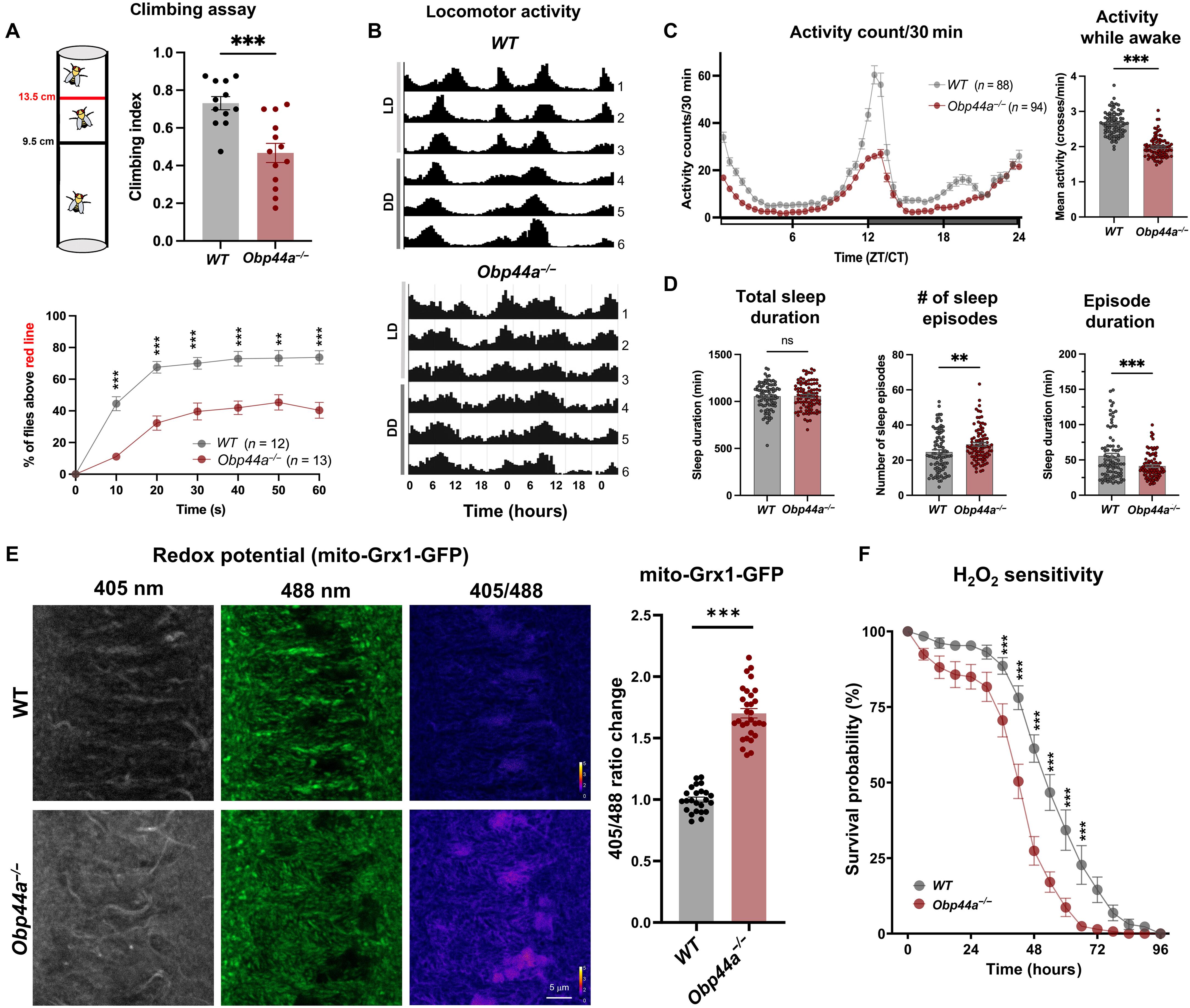
Glia-derived noncanonical fatty acid binding protein modulates brain lipid storage and clearance
Glia-derived secretory factors are essential for brain development, physiology, and homeostasis, with their dysfunction linked to a variety of neurological disorders. Through genetic and biochemical approaches, we identified odorant binding protein 44a (Obp44a), a noncanonical α-helical fatty acid binding protein (FABP) highly expressed in Drosophila central nervous system glia. Obp44a binds long-chain fatty acids and shuttles between glia and neurons, acting as a secretory lipid chaperone and scavenger to support lipid storage, efflux, and redox homeostasis. Notably, Obp44a is recruited to apoptotic cells and injured axons, especially when glial engulfment is impaired, demonstrating its role in lipid waste management and clearance of cellular debris during development and in pathological states. Our findings highlight FABPs’ importance in regulating brain lipid dynamics and neuronal response to stress and injury. By visualizing FABP function in vivo, this study provides insights into how defective lipid regulation may contribute to neuronal stress and disease progression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


