推进靶向癌症纳米药物的工程设计策略
IF 66.8
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
工程纳米粒子通过封装和输送治疗和诊断试剂,极大地扩展了癌症治疗,否则会受到不良药代动力学和毒性的限制,从而靶向肿瘤细胞。利用我们对肿瘤微环境的进一步了解,纳米医学已经扩展到与肿瘤发生有关的关键组织和细胞,如免疫细胞和基质细胞,以提高效力并进一步减轻脱靶毒性。为了设计克服人体生理障碍进入肿瘤的纳米载体,该领域已经探索了更广泛的给药途径和纳米颗粒设计原则,而不仅仅是增强渗透和保留效果。这篇综述探讨了纳米颗粒的非共价表面修饰的优势,以及其他表面修饰,以调节纳米颗粒从注射部位运输到肿瘤和淋巴组织,到靶细胞,并最终其亚细胞命运。利用静电或其他非共价技术,纳米颗粒表面可以用天然和合成的大分子修饰,从而实现高度精确的细胞和组织运输。合理的设计还可以减少免疫系统的检测和清除,延长半衰期,这是最大限度地提高治疗药物疗效的关键。最后,我们概述了癌症纳米医学如何通过结合新的筛选技术,计算方法和患者水平数据来设计有效的靶向治疗而继续发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。

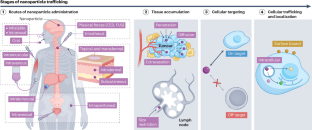
Advancing engineering design strategies for targeted cancer nanomedicine
Engineered nanoparticles have greatly expanded cancer treatment by encapsulating and delivering therapeutic and diagnostic agents, otherwise limited by poor pharmacokinetics and toxicity, to target tumour cells. Leveraging our increased understanding of the tumour microenvironment, nanomedicine has expanded to additionally target key tissues and cells implicated in tumorigenesis, such as immune and stromal cells, to improve potency and further mitigate off-target toxicities. To design nanocarriers that overcome the body’s physiological barriers to access tumours, the field has explored broader routes of administration and nanoparticle design principles, beyond the enhanced permeation and retention effect. This Review explores the advantages of non-covalent surface modifications of nanoparticles, along with other surface modifications, to modulate nanoparticle trafficking from the injection site, into tumour and lymphoid tissues, to the target cell, and ultimately its subcellular fate. Using electrostatic or other non-covalent techniques, nanoparticle surfaces can be decorated with native and synthetic macromolecules that confer highly precise cell and tissue trafficking. Rational design can additionally minimize detection and clearance by the immune system and prolong half-life — key to maximizing efficacy of therapeutic cargos. Finally, we outline how cancer nanomedicine continues to evolve by incorporating learnings from novel screening technologies, computational approaches and patient-level data to design efficacious targeted therapies. Nanoparticle surfaces can be engineered for targeted delivery of cancer therapies. In this Review, Gomerdinger, Nabar and Hammond outline the role of surface chemistry at all levels of nanoparticle trafficking, from administration route, to tissue accumulation, cellular targeting and ultimately subcellular localization. They emphasize the utility of non-covalent surface modifications for improving stealth and targeting abilities of nanoparticles for cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Reviews Cancer
医学-肿瘤学
CiteScore
111.90
自引率
0.40%
发文量
97
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Cancer, a part of the Nature Reviews portfolio of journals, aims to be the premier source of reviews and commentaries for the scientific communities it serves. The correct abbreviation for abstracting and indexing purposes is Nat. Rev. Cancer. The international standard serial numbers (ISSN) for Nature Reviews Cancer are 1474-175X (print) and 1474-1768 (online). Unlike other journals, Nature Reviews Cancer does not have an external editorial board. Instead, all editorial decisions are made by a team of full-time professional editors who are PhD-level scientists. The journal publishes Research Highlights, Comments, Reviews, and Perspectives relevant to cancer researchers, ensuring that the articles reach the widest possible audience due to their broad scope.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


