NPTX下调引起的印痕网络紊乱是衰老相关情境恐惧记忆缺陷的基础
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
储存情景记忆的印迹细胞被分配到不同的神经元群中。然而,这些组合如何保持其稳定性以驱动精确的记忆表达,以及它们的不稳定性是否导致与衰老相关的记忆缺陷,仍然是难以捉摸的。本研究表明,在情境恐惧记忆巩固过程中,齿状回Fos转录依赖集合(F-RAM)中的神经元戊烷素1 (NPTX1)促进了恐惧情境下的记忆表达。NPTX1促进Kv7.2通道介导的印痕细胞高兴奋性抑制,从而限制这些细胞对内嗅皮层兴奋性输入的反应。同时,NPTX2增强了parvalbumin+ (PV+)中间神经元对Npas4转录依赖集合(N-RAM)的周围抑制作用,从而防止了恐惧记忆的过度概括。Kv7.2通道的药理激活或PV+中间神经元的化学发生激活修复了由印迹特异性NPTX缺失引起的记忆缺陷。老龄小鼠情境恐惧记忆精度下降,DG印迹细胞NPTX表达下降。在F-RAM集合中过表达NPTX1或在N-RAM集合中过表达NPTX2的ampar结合域可改善情境恐惧记忆缺陷。这些发现阐明了NPTX1和NPTX2的协同作用可以防止印痕网络变得过度活跃,并提供了印痕网络不稳定与衰老相关的情境恐惧记忆缺陷之间的因果联系。本文章由计算机程序翻译,如有差异,请以英文原文为准。

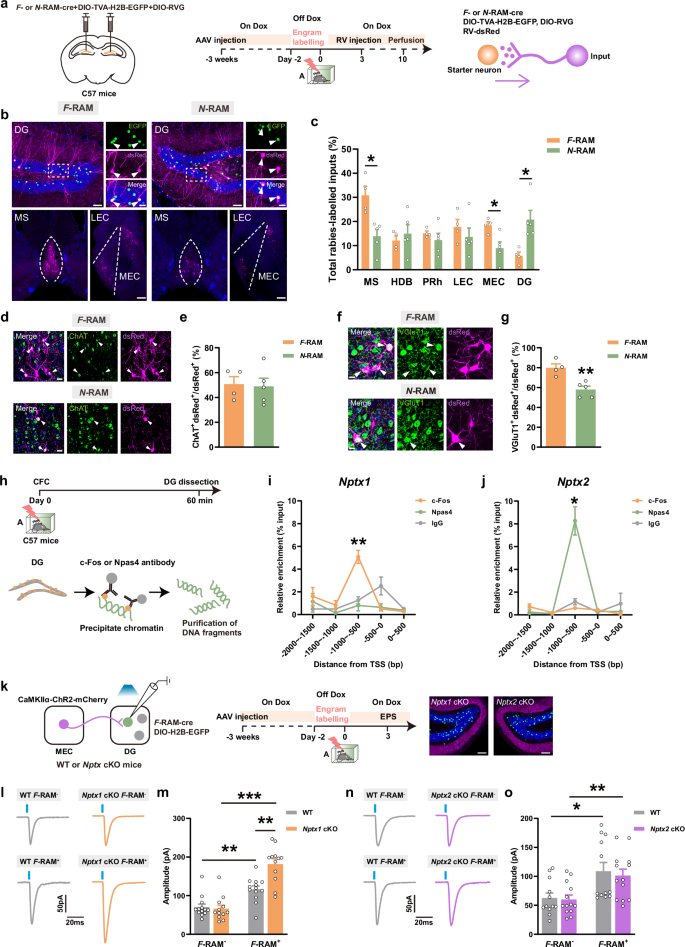
Disturbed engram network caused by NPTX downregulation underlies aging-related contextual fear memory deficits
Engram cells storing episodic memories are allocated to separate neuronal ensembles. However, how these ensembles maintain their stability to drive precise memory expression, and whether their destabilization contributes to aging-related memory deficits, remain elusive. Here, we show that during contextual fear memory consolidation, neuronal pentraxin 1 (NPTX1) in Fos transcription-dependent ensemble (F-RAM) of the dentate gyrus (DG) promotes memory expression in the fear context. NPTX1 facilitates Kv7.2 channel-mediated inhibition of engram cell hyperexcitability, thereby restricting the response of these cells to excitatory inputs from medial entorhinal cortex. Meanwhile, NPTX2 enhances the perisomatic inhibition of Npas4 transcription-dependent ensemble (N-RAM) by parvalbumin+ (PV+) interneurons, thereby preventing fear memory overgeneralization. Pharmacological activation of Kv7.2 channels or chemogenetic activation of PV+ interneurons repaired memory deficits caused by engram-specific NPTX depletion. Contextual fear memory precision and NPTX expression in DG engram cells were decreased in aged mice. Overexpressing NPTX1 in F-RAM ensemble or the AMPAR-binding domain of NPTX2 in N-RAM ensemble rescued contextual fear memory deficits. These findings elucidate that the coordination of NPTX1 and NPTX2 prevents engram ensembles from becoming hyperactive and provide a causal link between engram network destabilization and aging-related contextual fear memory deficits.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


