低氧利用近红外治疗纳米医学使成像引导肿瘤诊断和光热治疗。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
治疗性纳米医学已经成为一种很有前途的方法,它将治疗和诊断整合到一个单一的纳米平台中,从而彻底改变癌症治疗。利用七甲基菁菁染料的近红外荧光发射和光热转换效应,我们以荧光光敏剂(Cy7-NO2)为基础,在近红外光照射下具有较高的光热转换效率(η = 31.4%),开发了一种近红外治疗纳米药物(Cy7-NO2- nm),实现了肿瘤的协同诊断和光热治疗。在缺氧肿瘤组织和细胞中,当过表达的硝基还原酶存在时,Cy7-NO2被还原并转化为含有相同荧光团(Cy7-NH2)的非荧光对应物,从而可以利用它来感知肿瘤缺氧。Cy7-NO2对肿瘤微环境敏感的光学行为使纳米药物能够区分不同大小的肿瘤病变,实现尺寸依赖性肿瘤诊断。同时,得到的Cy7-NH2也是光热剂,光热转化效率接近Cy7-NO2。因此,在响应肿瘤缺氧暴露肿瘤病变前后,纳米药物在近红外光照射下对癌组织和细胞产生不间断的光疗作用。本研究为单分子纳米药物在影像引导下的协同肿瘤诊断和光治疗提供了一种简单而有效的治疗策略。意义声明:本综述强调了线粒体自噬在癌症中的治疗相关性,重点关注其在线粒体质量控制和肿瘤调节中的选择性作用。鉴于在精确的线粒体自噬调节方面的挑战,我们强调了基于纳米技术的递送系统作为一个有前途的解决方案的出现。综述涵盖了线粒体自噬机制、相关途径、检测技术、线粒体自噬调节剂和纳米颗粒策略,包括独立和组合。它进一步讨论了转化机会和技术障碍,为纳米医学如何实现靶向有丝分裂干预以改善癌症治疗提供了一个简明、综合的视角。本文章由计算机程序翻译,如有差异,请以英文原文为准。
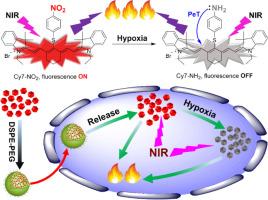
A hypoxia-leveraged near-IR theranostic nanomedicine enables imaging-guided tumor diagnosis and photothermal therapy
Theranostic nanomedicine has emerged as a promising approach that integrates both therapy and diagnostics into a single nanoplatform in revolutionizing cancer treatment. Taking advantage of the near-IR fluorescence emission and photothermal conversion effects of heptamethine cyanine dyes, we have developed a near-IR theranostic nanomedicine (Cy7-NO2![]() NM) based on a fluorescent photosensitizer (Cy7-NO2) with a high photothermal conversion efficiency (η = 31.4 %) upon near-IR light irradiation, attaining synergistic tumor diagnosis and photothermal therapy. In the presence of overexpressed nitroreductase in hypoxic tumor tissues and cells, Cy7-NO2 is reduced and converted into a nonfluorescent counterpart containing the same fluorophore (Cy7-NH2), which thereby can be leveraged to sense tumor hypoxia. The sensitive optical behaviors of Cy7-NO2 in response to the tumor microenvironment enable the nanomedicine to differentiate tumor lesions of different sizes, achieving size-dependent tumor diagnosis. Meanwhile, the resulting Cy7-NH2 is a photothermal agent too, as nearly efficient as Cy7-NO2 in photothermal conversion. Therefore, before and after responding to the tumor hypoxia to expose the tumor lesions, the nanomedicine exerts uninterrupted phototherapeutic effects on cancerous tissues and cells under near-IR light irradiation. This study provides a simple but effective theranostic strategy to develop single-molecule nanomedicine for synergistic imaging-guided tumor diagnosis and phototheranostics.
NM) based on a fluorescent photosensitizer (Cy7-NO2) with a high photothermal conversion efficiency (η = 31.4 %) upon near-IR light irradiation, attaining synergistic tumor diagnosis and photothermal therapy. In the presence of overexpressed nitroreductase in hypoxic tumor tissues and cells, Cy7-NO2 is reduced and converted into a nonfluorescent counterpart containing the same fluorophore (Cy7-NH2), which thereby can be leveraged to sense tumor hypoxia. The sensitive optical behaviors of Cy7-NO2 in response to the tumor microenvironment enable the nanomedicine to differentiate tumor lesions of different sizes, achieving size-dependent tumor diagnosis. Meanwhile, the resulting Cy7-NH2 is a photothermal agent too, as nearly efficient as Cy7-NO2 in photothermal conversion. Therefore, before and after responding to the tumor hypoxia to expose the tumor lesions, the nanomedicine exerts uninterrupted phototherapeutic effects on cancerous tissues and cells under near-IR light irradiation. This study provides a simple but effective theranostic strategy to develop single-molecule nanomedicine for synergistic imaging-guided tumor diagnosis and phototheranostics.
Statement of significance
- A new single-molecule theranostic nanomedicine strategy integrating near-IR fluorescence imaging-guided tumor diagnosis and photothermal therapy was developed and evaluated in vitro and in vivo.
- •The synergistic diagnostic and therapeutic functions of the nanomedicine rely on a hypoxia-sensitive fluorescent photosensitizer, which exhibits sensitive optical responses toward the hypoxic environment of tumor tissues, enabling the nanomedicine to differentiate tumor lesions of different sizes and thus achieving size-dependent tumor diagnosis.
- •The photosensitizer has a high photothermal conversion efficiency upon near-IR light irradiation and thus can serve as a photothermal therapeutic agent for cancer treatment. Before and after detecting tumors, the nanomedicine retains its photothermal efficiency and keeps exerting photothermal effects on tumor lesions uninterruptedly.
- •Our research provides a simple but effective theranostic strategy to develop single-molecule nanomedicine for synergistic imaging-guided tumor diagnosis and phototheranostics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


