工程蚕丝-蜘蛛复合丝的结构和力学性能。
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
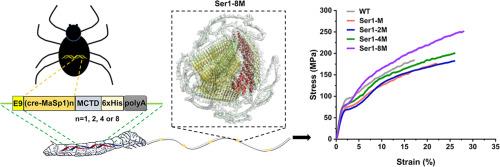
蜘蛛丝具有良好的力学性能和生物相容性,在生物材料和医学应用方面具有重要的潜力。然而,蜘蛛很难大规模饲养,而且直接从蜘蛛身上获取丝蛋白效率低下且价格昂贵。解决这些挑战的一个有希望的策略是在转基因蚕中表达蜘蛛丝蛋白。在本研究中,采用转录激活因子样效应核酸酶(TALEN)介导的基因组靶向编辑,分别将黑寡妇蜘蛛的cre-MaSp1基因的1-、2-、4-和8倍重复序列融合到丝胶蛋白1基因上。AlphaFold 3结构预测和红外光谱分析表明,复合丝蛋白的β-sheet和螺旋含量随着融合cre-MaSp1重复次数的增加而逐渐增加。力学性能测试表明,含有8倍cre-MaSp1/Ser1融合蛋白的蚕蛛复合丝的最大应力和最大应变分别比野生型高39.4%和62.2%,在所有品系中表现最佳。本研究为丝胶蛋白修饰提供了新的见解,并进一步证实了含有大量重复序列的cre-MaSp1基因的表达可以改善蚕丝的力学性能。意义陈述:蚕丝是一种天然蛋白质纤维,蚕丝性能的提高是一个长期关注的问题。本研究旨在通过对蚕丝蛋白进行靶向修饰,赋予蚕丝功能蛋白,从而改善蚕丝的力学性能。通过talen介导的同源定向重组,将黑寡妇蜘蛛4个不同重复的cre-MaSp1基因分别融合到内源性Ser1中。融合蛋白成功表达并分泌到茧壳中。拉伸试验表明,8次cre-MaSp1重复显著提高了蚕丝的最大应力和最大应变,分别比野生型提高了39.4%和62.2%。我们的工作为改善蚕丝性能提供了见解,并扩大了蚕丝腺生物反应器的潜在应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Structural and mechanical properties of engineered silkworm-spider composite silk
Spider silk demonstrates significant potential for biomaterials and medicinal applications owing to its favorable mechanical properties and biocompatibility. However, spiders are difficult to raise on a large scale, and obtaining silk proteins directly from spiders is inefficient and expensive. A promising strategy for addressing these challenges involves expressing spider silk proteins in transgenic silkworms. In this study, transcription activator-like effector nuclease (TALEN)-mediated genome-targeted editing was employed to separately fuse 1-, 2-, 4-, and eightfold repeats of the cre-MaSp1 gene from black widow spiders to the sericin 1 gene. AlphaFold 3 structure prediction and infrared spectroscopy showed that the β-sheet and helix contents of the composite silk proteins progressively increased with the increase in the number of fused cre-MaSp1 repeats. Mechanical property testing showed that the maximum stress and maximum strain of the silkworm-spider composite silk containing the eightfold cre-MaSp1/Ser1 fusion protein were 39.4 % and 62.2 % higher than those of the wild-type, respectively, representing the best performance among all the lines. This study provides insights into sericin modification and further confirms that the expression of the cre-MaSp1 gene harboring a large number of repeats can improve the mechanical properties of silkworm silk.
Statement of significance
Silkworm silk is a kind of natural protein fiber, and the improvement of silk performance is a long-term focus. This study aims to improve the mechanical properties of silk by endowing it with functional proteins through targeted modification of silk proteins. Four different repeats of cre-MaSp1 gene from black widow spiders separately fused into endogenous Ser1 by TALEN-mediated homology-directed recombination. The fusion proteins were successfully expressed and secreted into the cocoon shell. Tensile testing indicated that eightfold cre-MaSp1 repeats significantly increased the maximum stress and strain of the composite silk by 39.4 % and 62.2 % over the wild-type, respectively. Our work provides insights into improving silk properties and expands the potential applications of the silkworm silk gland bioreactor.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


