组织病理学评估作为韩国黄毒蛇毒抗出血效价检测的补充方法的初步探索。
IF 2.4
4区 医学
Q2 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
韩国抗蛇毒血清的抗出血效价检测依赖于主观的视觉损伤测量,限制了客观性和可重复性。这项初步研究评估了组织病理学评分作为一种补充方法。在兔模型中,出血和胶原蛋白破坏的半定量评估显示与抗蛇毒血清疗效有很强的剂量依赖性。这些发现表明,组织病理学评估可以补充传统的检测方法,提高可靠性,并支持未来朝着客观、标准化和合乎道德规范的抗蛇毒血清质量控制的努力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
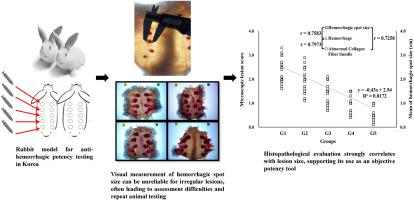
A preliminary exploration of histopathological assessment as a complementary approach to anti-hemorrhagic potency testing of Gloydius antivenom in Korea
Anti-hemorrhagic potency testing for Gloydius antivenom in Korea relies on subjective visual lesion measurement, limiting objectivity and reproducibility. This pilot study evaluated histopathological scoring as a complementary approach. In a rabbit model, semiquantitative assessment of hemorrhage and collagen disruption showed strong, dose-dependent correlations with antivenom efficacy. These findings suggest that histopathological evaluation can supplement traditional assays, enhancing reliability and supporting future efforts toward objective, standardized, and ethically refined antivenom quality control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Toxicon
医学-毒理学
CiteScore
4.80
自引率
10.70%
发文量
358
审稿时长
68 days
期刊介绍:
Toxicon has an open access mirror Toxicon: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review. An introductory offer Toxicon: X - full waiver of the Open Access fee.
Toxicon''s "aims and scope" are to publish:
-articles containing the results of original research on problems related to toxins derived from animals, plants and microorganisms
-papers on novel findings related to the chemical, pharmacological, toxicological, and immunological properties of natural toxins
-molecular biological studies of toxins and other genes from poisonous and venomous organisms that advance understanding of the role or function of toxins
-clinical observations on poisoning and envenoming where a new therapeutic principle has been proposed or a decidedly superior clinical result has been obtained.
-material on the use of toxins as tools in studying biological processes and material on subjects related to venom and antivenom problems.
-articles on the translational application of toxins, for example as drugs and insecticides
-epidemiological studies on envenoming or poisoning, so long as they highlight a previously unrecognised medical problem or provide insight into the prevention or medical treatment of envenoming or poisoning. Retrospective surveys of hospital records, especially those lacking species identification, will not be considered for publication. Properly designed prospective community-based surveys are strongly encouraged.
-articles describing well-known activities of venoms, such as antibacterial, anticancer, and analgesic activities of arachnid venoms, without any attempt to define the mechanism of action or purify the active component, will not be considered for publication in Toxicon.
-review articles on problems related to toxinology.
To encourage the exchange of ideas, sections of the journal may be devoted to Short Communications, Letters to the Editor and activities of the affiliated societies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


