骨肉瘤治疗中免疫微环境重建与免疫化疗的智能药物共给药平台
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
免疫治疗已成为一种新的肿瘤抑制策略,但在骨肉瘤治疗中,其有效性受到免疫抑制肿瘤微环境(TME)的限制。因此,一系列以免疫激活为重点的治疗方法已被引入,作为与免疫治疗的联合治疗,特别是具有诱导免疫原性细胞死亡(ICD)能力的化疗。在这项研究中,我们设计了一个纳米平台(chchanpdox),将阿霉素(DOX)和顺铂(CDDP)共同递送到肿瘤部位,协同杀死恶性细胞。由于DOX诱导ICD的能力,通过肿瘤特异性抗原暴露和树突状细胞成熟重建了TME的免疫原性。此外,chchanpdox治疗增加了肿瘤对抗程序性细胞死亡-1 (aPD-1)的敏感性。我们的体内实验结果表明,chchanpdox和aPD-1联合使用不仅能成功抑制肿瘤生长,还能抑制肿瘤复发和肺转移。抗肿瘤作用的增强可归因于激活的免疫反应和建立的免疫记忆。这种组合可能是骨肉瘤的潜在免疫化疗选择。本文章由计算机程序翻译,如有差异,请以英文原文为准。
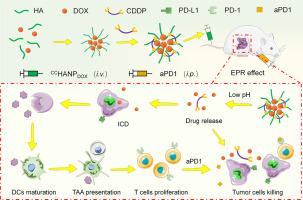
Intelligent drug codelivery platform for immune microenvironment reconstruction and immunochemotherapy in osteosarcoma treatment
Immunotherapy has emerged as a new strategy for tumor suppression, but its effectiveness is restricted by the immunosuppressive tumor microenvironment (TME) in the context of osteosarcoma treatment. Therefore, a series of therapies focused on immune activation have been introduced as combination treatments with immunotherapy, especially chemotherapies with the ability to induce immunogenic cell death (ICD). In this study, we designed a nanosized platform (CCHANPDOX) to codeliver doxorubicin (DOX) and cisplatin (CDDP) to the tumor site for synergistic killing of malignant cells. Due to the ability of DOX to induce ICD, the immunogenicity of the TME was reconstructed via tumor-specific antigen exposure and dendritic cell maturation. Furthermore, CCHANPDOX treatment increased the sensitivity of tumors to anti-programmed cell death-1 (aPD-1). Our in vivo results demonstrated that the combination of CCHANPDOX and aPD-1 not only successfully depressed tumor growth but also inhibited tumor recurrence and lung metastasis. The enhanced anti-tumor effect can be attributed to the activated immune response and established immune memory. This combination may be a potential immunochemotherapy option for osteosarcoma.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


